Abstract
Although the effects of seasonality on syphilis have been discussed previously, no previous study has evaluated the seasonality of syphilis incidence by sex and age group. We examined the seasonality of syphilis incidence by sex and age group in Korea from 2011 to 2019. The incidence of syphilis was calculated on the basis of Korea Diseases Control and Prevention Agency data, and an autoregressive integrated moving average (ARIMA) model and seasonal and trend decomposition using Loess were used to analyze the seasonality of the incidence in relation to epidemiological factors. The annual age-standardized incidence rates of primary, secondary, and congenital syphilis were 21.1, 8.8, and 64.0 cases/million persons, respectively, from 2011 to 2019. The highest incidence rates for primary and secondary syphilis were observed among those aged 20 to 29, 13 to 19, and 30 to 49 years, but not among the lower age groups. In analyses based on the ARIMA model, all univariate time series showed the highest goodness-of-fit results with ARIMA for primary syphilis (1,1,2), secondary syphilis (1,1,1), and congenital syphilis (0,1,2) (2,0,0) models. This study suggests that the incidence of secondary syphilis shows a summer seasonality for males and the highest incidence rate in the 20 to 29-year age group for both males and females in Korea. Public health action is needed to prevent an increase in syphilis incidence associated with sex, age group, and seasonal patterns.
Keywords: epidemiology, seasonality, sexually transmitted disease, syphilis
1. Introduction
Syphilis is a chronic and systemic infectious disease caused by Treponema pallidum.[1,2] Patients may be aware of the infection at the time of contact with the virus, and the condition is treatable. It is primarily transmitted through sexual contact, and patients may show a variety of clinical symptoms according to the disease stage. In the primary and secondary stages, abnormalities in cerebrospinal fluid and T pallidum invasion of the central nervous system are common. Although most patients are often asymptomatic, they may develop deafness and acute syphilitic meningitis with cranial nerve palsies. Over several years after the initial infection, gummas of the skin, musculoskeletal system, internal organs, or central nervous system develop in the form of meningovascular syphilis.[3] Penicillin G is mainly used to treat syphilis, while some newer antibiotics have also been studied for the treatment of syphilis.[2–5]
The occurrence of syphilis drastically reduced after the 1940s, when penicillin was developed; however, it subsequently increased again due to an increase in human immunodeficiency virus infection and drug abuse,[2,6] and syphilis cases continue to rise.[6–8] The incidence rates of primary and secondary syphilis were 2.12 per 100,000 in 2000 and climbed to 9.5 per 100,000 in 2017, respectively.[6] Estimates of the global male and female prevalence were 0.58 and 0.56, respectively, in 2016, and they increased to 0.58 and 0.56, respectively, in 2020.[8]
The main transmission route is direct contact with bodily fluids or exposed lesions of patients with syphilis during sexual intercourse. In rare cases, infection occurs during a blood transfusion or via vertical transmission through the placenta in a pregnant woman.[9,10] According to the World Health Organization, a million or more cases of sexually transmitted diseases (STDs) occur per day worldwide, and each year 376 million or more individuals are infected with 1 of the 4 major STDs, i.e., chlamydia infection, gonorrhea, syphilis, and trichomoniasis. Due to syphilis, at least 300,000 fetuses and newborn babies die annually, and an additional 200,000 or more infants are at risk of early death.[8,11] If left untreated, syphilis can cause complications in various organs, including the central nervous system. In cases of syphilis co-occurring with other diseases causing genital ulcers, the likelihood of human immunodeficiency virus infection and transmission increases.[8]
In South Korea, syphilis was classified into reportable infectious disease group 3 and managed under the Infectious Disease Surveillance System from 2011 to 2019. In 2020, it was re-classified into reportable infectious disease group 4; since then, it has been managed under the sentinel surveillance system.[12] In the latter system, it is impossible to collect data on epidemiological characteristics.
A Chinese research team had previously conducted a time-series analysis using syphilis cases reported between 2005 and 2012 and found that the occurrence increased in the summer.[13] However, very few studies have been conducted on the seasonality of syphilis. This study aimed to examine the seasonality of syphilis using autoregressive integrated moving average (ARIMA) modeling and attempted to predict future trends applying exponential smoothing and seasonal and trend decomposition using Loess, based on the data collected through the infectious disease surveillance system during 2011 to 2019 in South Korea. By defining the seasonality of syphilis in mandatory weekly surveillance data, preventive measures can be devised.
2. Materials and methods
2.1. Data collection
The 2011 to 2019 syphilis data were reported through the Infectious Disease Surveillance System and managed by the Korea Centers for Disease Control and Prevention (KCDC). The cases were reported by healthcare institutions and transferred to public health centers in the corresponding regions. This information was reported to the KCDC by public health centers.[12] After a review by the KCDC, the reports were finally available for statistical analysis.[12] The Mandatory Surveillance System required obligatory reporting of syphilis cases to health centers without delay from 2011 to 2019. The incidence data for primary, secondary, and congenital syphilis in South Korea from 2011 to 2019 were used in this study. The type of syphilis was classified on the basis of specific symptoms and diagnostic tests (dark-field microscopy, non-treponemal test, and treponemal-specific test). The collected data included year, syphilis type, age, sex, date of onset, date of reporting, and occupation.
For population data, statistics on the resident registration population available in the national database of Statistics Korea were used. Based on the size of the resident registration population per year, sex, and age group in 2011 to 2019, incidence and age-standardized incidence rates were estimated.[14]
This cross-sectional study was approved by the KCDC Institutional Review Board (IRB No. 2020-05-04-P-A). The need to obtain the informed consent was waived by the IRB due to the retrospective nature of the study.
2.2. Statistical analysis
For each syphilis type, the incidence and age-standardized incidence rates were estimated on the basis of epidemiological factors. For primary and secondary syphilis, the base population was defined as the entire 2015 resident registration population to estimate the 2011 to 2019 age-standardized incidence rates by sex. The incidence of congenital syphilis was estimated by including ages below 1 year (year 0). At ages other than 0 years, 4 patients had congenital syphilis.
The variables used in the analysis of epidemiological factors were year, syphilis type, sex, age, and date of the report. Syphilis was classified into primary syphilis, secondary syphilis, and congenital syphilis. Individuals manifesting clinical symptoms compatible with the diagnosis based on the corresponding clinical criteria and showing infection with the pathogen based on the diagnostic test criteria were categorized into the respective groups. If the same individual was reported more than once within a year, the patient was regarded as being continuously treated since the earliest report and was counted as a single case. Age was categorized into the following groups according to lifespan stages: 0 to 5 years (infancy and early childhood), 6 to 12 years (school-age childhood), 13 to 19 years (adolescence), 20 to 29 years (young adulthood), 30 to 49 years (early middle adulthood), 50 to 64 years (late middle adulthood), 65 to 84 years (late adulthood), and over 85 years (very late adulthood).[15]
Temporal trend analysis was performed for weekly occurrences during the 9-year period. The normality and autocorrelation of the time-series data were examined, and the fitness of the data was assessed by performing ARIMA modeling.[16] In addition, a future trend was predicted by applying exponential smoothing and seasonal and trend decomposition using Loess under the assumption of non-normality.[17,18] Microsoft Excel was used to analyze the occurrence of syphilis by epidemiological factors, and R-4.2.1 for Windows (R Foundation for Statistical Computing, Vienna, Austria) was used to perform temporal analysis.[19]
3. Results
3.1. Distribution of syphilis incidence
The total number of cases reported during 2011 to 2019 was 8521 for primary syphilis, 3550 for secondary syphilis, and 235 for congenital syphilis. Unlike congenital syphilis, primary and secondary syphilis occurred more frequently in men than in women (n = 5512 [64.69%] in primary syphilis and n = 2326 [65.52%] in secondary syphilis). The median age was approximately 48 years (13–100 years) in primary syphilis, with more cases in the age groups of 20 to 29 years (young adulthood) and 30 to 49 years (early middle adulthood). The median age of patients with secondary syphilis was approximately 61 years (13–97 years), again showing higher occurrences in the age groups of 20 to 29 and 30 to 49 years. Regional differences were not large in primary, secondary, or congenital syphilis. Regarding occupation, many cases of primary and secondary syphilis were classified as Others. Excluding those whose occupation was unidentified, syphilis showed higher occurrences in the unemployed people (n = 828 [9.72%] in primary syphilis and n = 358 [10.08%] in secondary syphilis) and employed people (n = 798 [9.37%] in primary syphilis and n = 315 [8.87%] in secondary syphilis). Most cases of congenital syphilis were reported by general hospitals, whereas primary and secondary syphilis cases were reported mainly by general hospitals and clinics (Table 1).
Table 1.
The demographic characteristics of cases of syphilis, 2011 to 2019.
| Syphilis | ||||
|---|---|---|---|---|
| Primary syphilis n (%) |
Secondary syphilis n (%) |
Congenital syphilis n (%) |
P value | |
| Sex | <.001 | |||
| Male | 5512 (64.69) | 2326 (65.52) | 116 (49.36) | |
| Female | 3009 (35.31) | 1224 (34.48) | 119 (50.64) | |
| Age | <.001 | |||
| Mean (Range) | 48.04 (13–100) | 60.99 (13–97) | 0.06 (0–8) | |
| 0–5 | 0 (0.00) | 0 (0.00) | 234 (99.57) | |
| 6–12 | 0 (0.00) | 0 (0.00) | 1 (0.43) | |
| 13–19 | 623 (7.31) | 281 (7.92) | - | |
| 20–29 | 2928 (34.36) | 1325 (37.32) | - | |
| 30–49 | 3357 (39.4) | 1265 (35.63) | - | |
| 50–64 | 1183 (13.88) | 478 (13.46) | - | |
| 65–84 | 387 (4.54) | 177 (4.99) | - | |
| 85≤ | 43 (0.50) | 24 (0.68) | - | |
| Administrative division | <.001 | |||
| Metropolitan cities | 4018 (47.15) | 1802 (50.76) | 105 (44.68) | |
| Provinces | 4503 (52.85) | 1748 (49.24) | 130 (55.32) | |
| Status of occupation | <.001 | |||
| Employed | 798 (9.37) | 315 (8.87) | - | |
| Unemployed | 828 (9.72) | 358 (10.08) | 235 (100.00) | |
| Commercial sex worker | 42 (0.49) | 4 (0.11) | - | |
| Healthcare worker | 5 (0.06) | 7 (0.20) | - | |
| Housekeeper | 237 (2.78) | 88 (2.48) | - | |
| Armed forces | 233 (2.73) | 75 (2.11) | - | |
| Inmate | 43 (0.50) | 4 (0.11) | - | |
| Student | 376 (4.41) | 201 (5.66) | - | |
| Unidentified | 5959 (69.93) | 2498 (70.37) | - | |
| Institution | <.001 | |||
| General hospital | 4416 (51.82) | 2573 (72.48) | 229 (97.45) | |
| Hospital | 1035 (12.15) | 263 (7.41) | 4 (1.70) | |
| Clinic | 2274 (26.69) | 573 (16.14) | 2 (0.85) | |
| Public health center | 707 (8.3) | 130 (3.66) | - | |
| Oriental medical institution | 22 (0.26) | 1 (0.03) | - | |
| Jail | 13 (0.15) | 5 (0.14) | - | |
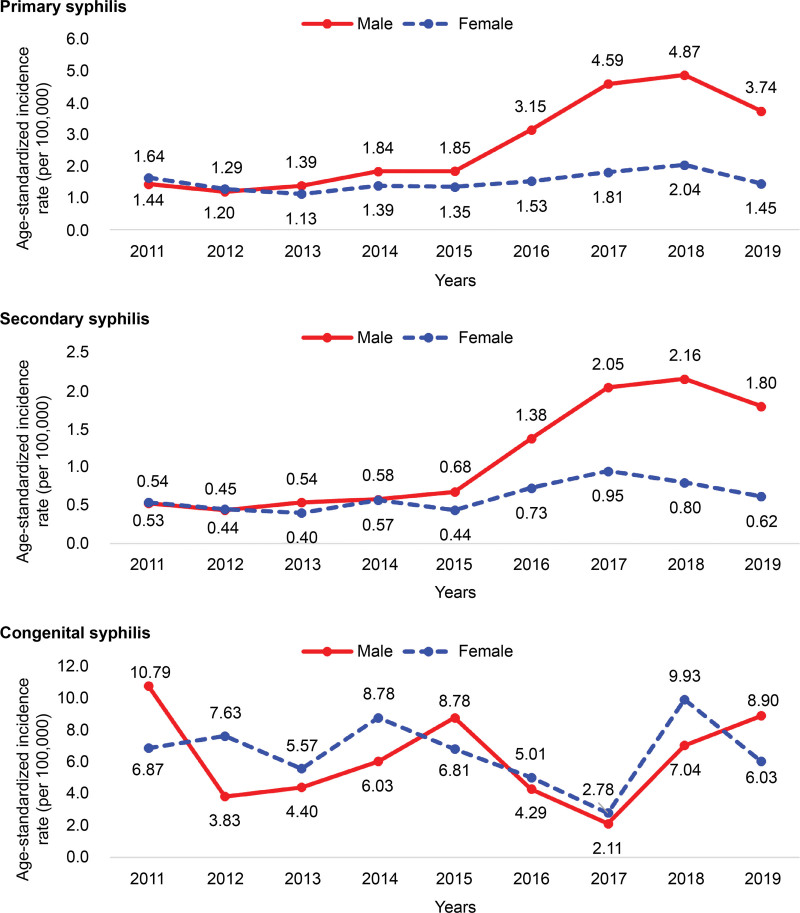
The annual age-standardized incidence of primary syphilis was 2.11 per 100,000 population. It showed an increasing trend from 2012 but reduced in 2019. In categorization by sex, the annual age-standardized incidence in men was 2.68 per 100,000 population, which was higher than that in women (1.52 per 100,000 population). The annual age-standardized incidence by year/sex was 4.87 per 100,000 population in men and 2.04 per 100,000 population in women, and in both sexes, it was the highest in 2018. The annual age-standardized incidence of secondary syphilis was 0.88 per 100,000 population and showed an increasing trend from 2012 to 2019, when it decreased. The annual age-standardized incidence was 1.13 per 100,000 population in men and higher than that in women (0.62 per 100,000 population). In categorization by year/sex, the age-standardized incidence was the highest in 2018 in men (2.16 per 100,000 population) and in 2017 in women (0.95 per 100,000 population). The annual incidence of congenital syphilis was 6.40 per 100,000 population. The incidence was the highest in 2018 (8.44 per 100,000 population). The annual age-standardized incidence by sex was 6.60 per 100,000 population in women and 6.20 per 100,000 population in men. By year and sex, men showed the highest age-standardized incidence in 2011 (10.79 per 100,000 population) and women in 2018 (9.93 per 100,000 population) (Fig. 1).
Figure 1.
Annual age-standardized incidence rate (per 100,000) of syphilis by sex and age, 2011 to 2019.
The annual incidence of primary syphilis was the highest (6.04 per 100,000 population) in men aged 20 to 29 years (young adulthood). By year/sex/age, the incidence in 2018 was the highest (11.20 per 100,000 population) in men aged 20 to 29 years (young adulthood). Likewise, the annual incidence of secondary syphilis estimated by sex/age was the highest (2.91 per 100,000 population) in men aged 20 to 29 years (young adulthood). By year/sex/age, the incidence was the highest (5.79 per 100,000 population) in men aged 20 to 29 years (young adulthood) in 2017 (Table 2).
Table 2.
Annual crude incidence rate (per 100,000) of primary and secondary syphilis by sex and age group, 2011 to 2019.
| Yr | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary syphilis | |||||||||||
| Sex | |||||||||||
| Male | Age | ||||||||||
| 13–19 | 1.12 | 1.15 | 0.89 | 1.41 | 0.95 | 1.69 | 1.63 | 2.52 | 1.33 | 1.38 | |
| 20–29 | 2.42 | 2.74 | 2.59 | 3.56 | 4.32 | 7.68 | 10.66 | 11.20 | 8.79 | 6.04 | |
| 30–49 | 1.85 | 1.39 | 1.80 | 2.22 | 2.17 | 3.40 | 5.69 | 5.65 | 4.61 | 3.16 | |
| 50–64 | 0.96 | 0.67 | 0.90 | 1.35 | 1.11 | 1.66 | 2.29 | 2.30 | 1.65 | 1.47 | |
| 65–84 | 0.37 | 0.09 | 0.25 | 0.24 | 0.38 | 1.36 | 1.48 | 2.48 | 1.41 | 0.97 | |
| 85≤ | 1.04 | 0.00 | 0.91 | 0.00 | 0.00 | 2.13 | 1.29 | 0.58 | 3.15 | 1.15 | |
| Female | Age | ||||||||||
| 13–19 | 2.39 | 2.08 | 1.35 | 1.74 | 1.33 | 1.64 | 1.98 | 2.57 | 1.44 | 1.84 | |
| 20–29 | 3.97 | 3.33 | 2.96 | 3.39 | 3.30 | 3.57 | 4.10 | 4.17 | 3.27 | 3.57 | |
| 30–49 | 1.46 | 1.14 | 1.18 | 1.26 | 1.39 | 1.34 | 1.64 | 1.75 | 1.26 | 1.37 | |
| 50–64 | 1.10 | 0.73 | 0.53 | 1.06 | 0.85 | 1.02 | 1.11 | 1.20 | 0.96 | 0.96 | |
| 65–84 | 0.13 | 0.03 | 0.00 | 0.03 | 0.09 | 0.71 | 0.86 | 1.57 | 0.88 | 0.51 | |
| 85≤ | 0.00 | 0.00 | 0.00 | 0.00 | 0.26 | 0.72 | 1.78 | 2.28 | 1.15 | 0.82 | |
| Total | 1.58 | 1.28 | 1.28 | 1.63 | 1.60 | 2.35 | 3.21 | 3.45 | 2.57 | 2.11 | |
| Secondary syphilis | |||||||||||
| Sex | |||||||||||
| Male | Age | ||||||||||
| 13–19 | 0.19 | 0.47 | 0.32 | 0.37 | 0.43 | 0.59 | 1.39 | 1.16 | 0.58 | 0.59 | |
| 20–29 | 0.83 | 0.75 | 1.37 | 1.22 | 1.75 | 3.91 | 5.79 | 5.74 | 4.62 | 2.91 | |
| 30–49 | 0.59 | 0.51 | 0.55 | 0.81 | 0.68 | 1.45 | 2.01 | 2.29 | 2.07 | 1.20 | |
| 50–64 | 0.45 | 0.33 | 0.35 | 0.23 | 0.46 | 0.64 | 0.94 | 0.97 | 0.79 | 0.59 | |
| 65–84 | 0.42 | 0.09 | 0.13 | 0.04 | 0.11 | 0.29 | 0.60 | 0.91 | 0.70 | 0.39 | |
| 85≤ | 1.04 | 0.00 | 0.00 | 0.00 | 0.00 | 1.42 | 1.29 | 0.58 | 1.58 | 0.74 | |
| Female | Age | ||||||||||
| 13–19 | 0.87 | 0.71 | 0.81 | 1.28 | 0.67 | 0.94 | 1.09 | 0.77 | 0.63 | 0.87 | |
| 20–29 | 1.20 | 1.19 | 0.92 | 1.47 | 1.14 | 1.42 | 2.22 | 1.62 | 1.56 | 1.42 | |
| 30–49 | 0.45 | 0.39 | 0.30 | 0.49 | 0.42 | 0.71 | 0.81 | 0.62 | 0.38 | 0.51 | |
| 50–64 | 0.40 | 0.24 | 0.27 | 0.19 | 0.17 | 0.52 | 0.57 | 0.64 | 0.45 | 0.39 | |
| 65–84 | 0.13 | 0.03 | 0.03 | 0.03 | 0.06 | 0.29 | 0.52 | 0.69 | 0.54 | 0.28 | |
| 85≤ | 0.00 | 0.00 | 0.00 | 0.00 | 0.26 | 0.24 | 0.89 | 1.03 | 0.77 | 0.43 | |
| Total | 0.54 | 0.45 | 0.47 | 0.58 | 0.56 | 1.06 | 1.51 | 1.49 | 1.21 | 0.88 | |
3.2. Seasonality of the incidence of syphilis by week and year, 2011 to 2019
An augmented Dickey–Fuller test was performed to examine the normality of the syphilis time-series data. The results showed that all primary and secondary syphilis data were normally distributed (lag order = 0) at a statistically significant level (Table 3).
Table 3.
Stationarity of the incidence of syphilis by wk and yr, 2011 to 2019.
| ADF | Lag order | P value | |
|---|---|---|---|
| Primary syphilis | −13.811 | 0 | <.001 |
| Male | −14.058 | 0 | <.001 |
| Female | −17.015 | 0 | <.001 |
| Secondary syphilis | −14.047 | 0 | <.001 |
| Male | −14.486 | 0 | <.001 |
| Female | −17.302 | 0 | <.001 |
| Congenital syphilis | −20.788 | 0 | <.001 |
ADF = Augmented Dickey–Fuller statistics.
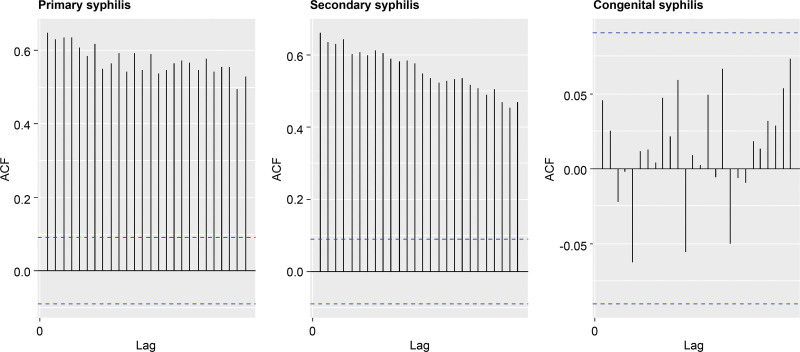
Autocorrelation functions were used to examine stationarity in the syphilis time-series data. For primary and secondary syphilis, the autocorrelation coefficients were positive at a significant level, whereas for congenital syphilis, white noise was observed (Fig. 2).
Figure 2.
Autocorrelation plot of the incidence of syphilis by wk and yr, 2011 to 2019.
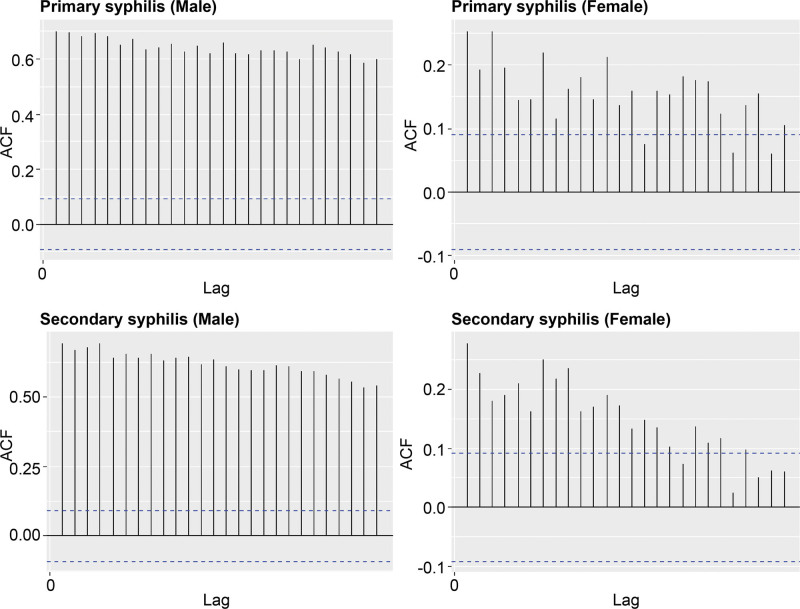
In primary and secondary syphilis in males, the autocorrelation coefficients were positive at a significant level, whereas in females, the autocorrelation coefficients were not significant (Fig. 3).
Figure 3.
Autocorrelation plot of the incidence of primary and secondary syphilis by wk and yr, 2011 to 2019.
ARIMA models were computed using the primary and secondary syphilis data (which showed a stable time-series pattern) and the congenital syphilis data (which showed an unstable time-series pattern). Model fitness testing based on the best models yielded the following results: Akaike information criterion was 3009.93 for primary syphilis, 2417.63 for secondary syphilis, and 1062.53 for congenital syphilis, while the corresponding Bayes information criteria were 3026.51, 2430.07, and 1083.26, respectively (Table 4).
Table 4.
The goodness-of-fit of the autoregressive integrated moving average model for the incidence of syphilis by wk and yr, 2011 to 2019.
| Log likelihood | AIC | BIC | |
|---|---|---|---|
| Primary syphilis (1,1,2) | −1500.96 | 3009.93 | 3026.51 |
| Male | −1355.43 | 2718.85 | 2735.44 |
| Female | −1176.70 | 2363.39 | 2384.12 |
| Secondary syphilis (1,1,1) | −1205.82 | 2417.63 | 2430.07 |
| Male | −1053.54 | 2111.07 | 2119.37 |
| Female | −935.5 | 1877.00 | 1889.44 |
AIC = Akaike information criterion, BIC = Bayes information criterion.
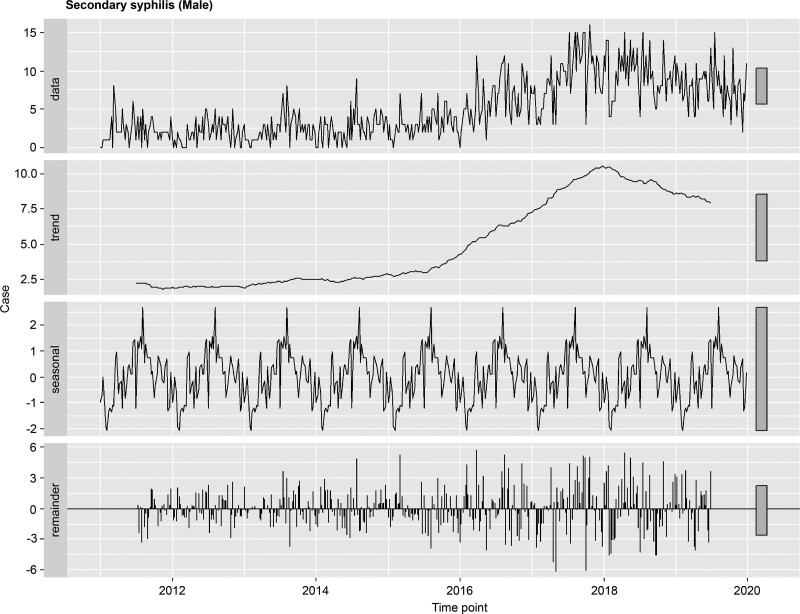
To examine seasonality, the 2011 to 2019 secondary syphilis time-series data were decomposed into trend, seasonal, and remainder components. For the weekly data of the 8-year period, a periodic seasonal component revealed that secondary syphilis primarily occurred in summer (Fig. 4).
Figure 4.
Time-series analysis for the incidence of secondary syphilis in males, 2011 to 2019.
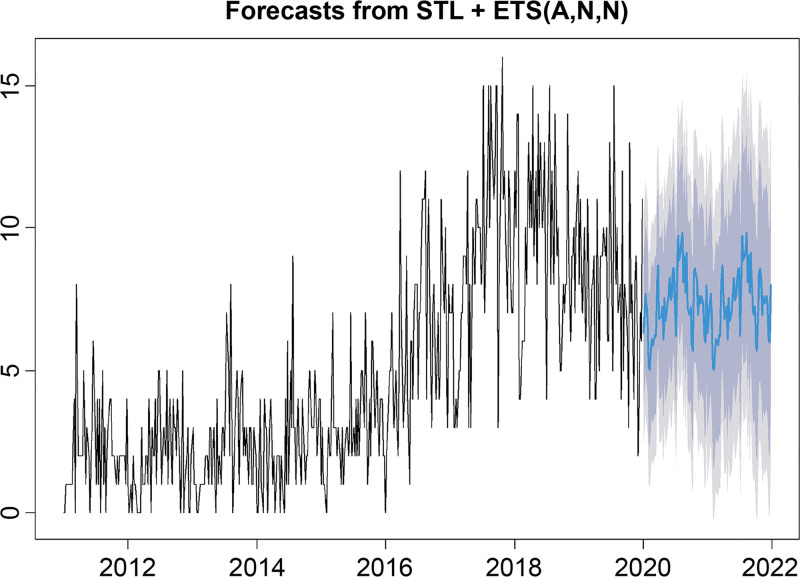
Future trend analysis of the occurrence of secondary syphilis predicted that it would reach a peak during the summer (Fig. 5).
Figure 5.
Prospective time series for forecasting the incidence of syphilis.
4. Discussion
This study estimated the occurrence of syphilis in South Korea, based on personal characteristics, using cases reported through the infectious disease surveillance system between 2011 and 2019 and provided a spatiotemporal analysis. The incidence of secondary syphilis demonstrated a summer seasonality for males, and the highest incidence rate was observed in the 20 to 29-year age group for both males and females in Korea.
To the best of our knowledge, this study is the first to assess the seasonality of syphilis by considering both normality and non-normality. This is also the first study to analyze data during the entire period in which syphilis cases were managed under an infectious disease surveillance system.[12] In South Korea, syphilis has been managed under this system since 2011, and a change was made in 2020 to use sentinel surveillance to manage syphilis cases. Thus, an epidemiological analysis using reported cases will be difficult to perform until the syphilis reporting system is changed back to the infectious disease surveillance system.
The annual occurrence rates of both primary and secondary syphilis steadily increased from 2015, peaking in 2018 and 2017, respectively, and then started to decrease. The annual occurrence rate was 2.11 per 100,000 population for primary syphilis, 0.88 per 100,000 population for secondary syphilis, and 6.40 per 100,000 population for congenital syphilis. Age-standardized incidence of both primary and secondary syphilis was more than 1.7 times higher in men than in women. In both types of syphilis, the incidence was the highest in young adults.
The pattern of a steady increase in primary and secondary syphilis cases until 2018 was also found in other countries. In Japan, the number of syphilis cases due to heterosexual contact in 2016 rapidly increased by 22.3 times in men and 40.4 times in women.[20] Although the occurrence of syphilis in Eastern Europe has decreased since the 2000s, the overall occurrence of syphilis has increased in Western Europe, as has its occurrence in male homosexual individuals and women. In Mexico, the occurrence of both acquired and congenital syphilis is increasing in men.[21,22] Thus, the increase in the prevalence of syphilis is a global phenomenon. However, the decrease in syphilis incidence in South Korea in 2019 is unusual. In 2020, the syphilis reporting system was changed from an infectious disease surveillance system to a sentinel surveillance system. Previously, after the Centers for Disease Control and Prevention/National Healthcare Safety Network modified the definition of catheter-associated urinary tract infection, the number of cases per 1000 catheter days decreased to 1.81 to 2.69, with an incidence rate ratio of 0.67 (95% CI: 0.48–0.93).[23] Therefore, changes in the operation of surveillance systems are likely to affect case reporting. Thus, the policy notice regarding the change to the sentinel surveillance system starting in 2020 may have influenced the reduction in case reporting in 2019.
From 2015, the incidence among men increased to more than twice than that among women. In Germany, syphilis outbreaks frequently occurred among homosexual men in 1997; moreover, 93.7% of the syphilis cases reported in Canada in 2015 involved men.[24,25] These findings of a large increase in the incidence of syphilis among men are in line with those of the current study. It is easy for homosexual men to meet sexual partners in bath houses and circuit parties, as well as through the Internet, and to frequently have sexual contact with new partners.[26–28] Additional research should be conducted to investigate whether this also applies to men in South Korea.
The occurrence rates of primary and secondary syphilis were the highest in young adults. One previous study reported that the age group of 25 to 29 years showed the highest incidence among all age groups in 2015 (21.2 per 100,000 population),[24] thus reflecting a pattern similar to the findings of the current study.
Excluding those whose occupation was unidentified, syphilis showed higher occurrences in the employed and unemployed people. A previous study reported that employment status resulted in no significant differences in the occurrence of syphilis,[29] thereby reflecting a pattern similar to the findings of the current study.
Temporal trend analysis confirmed the seasonality of secondary syphilis in males, showing that it primarily occurs in the summer. In a study conducted in China based on case reports from 2005 through 2012, time-series analysis showed that the occurrence was high in summer and low in winter. This finding is consistent with the current study finding that syphilis incidence was the highest in summer.[13] A previous study reported that during summer vacations, people tend to travel to places where STD outbreaks frequently occur and have sexual contact without condoms; reportedly, the incidence rate ratio of summer to winter in terms of syphilis incidence (summer:winter) was 5.48:5.03 in homosexual men, 2.46:2.31 in heterosexual men, and 1.83:1.72 in women.[30] In addition, with increasing sexual activity, sexual intercourse without the use of a condom was also greater during summer vacations.[31] The seasonality of syphilis is closely linked to sexual activity patterns in the population according to the season, indicating the need for research on sexual activity patterns.
This study has some limitations. First, because syphilis is an infectious disease reported through the infectious disease surveillance system, case reports were made passively by healthcare institution employees, and the data may not reflect the total number of patients with syphilis. Therefore, the occurrence rate of syphilis could not be accurately estimated. However, it is difficult to assume that the volume of unreported cases was larger in certain periods than in others; thus, nondifferential misclassification is assumed to occur because of missing reports. Accordingly, if the actual number of syphilis cases had been reported, the trend would have been similar, and the seasonality would have been similar or even clearer than the current findings.[32] Second, accurate data regarding patients’ occupations and potential transmission routes were not obtained, precluding an exploration of the risk levels pertaining to these factors. However, future studies can be expected to utilize the candidate risk groups identified in this study to explore high-risk groups.
Syphilis can remain latent from 10 days up to 3 months, and penicillin is used to treat it.[1,4,5] If healthcare institutions report a case after the patient presenting symptoms are treated, estimation of the source of infection and the date of symptom occurrence due to latency is difficult. However, treatments are available, and if syphilis is cured, it cannot be transmitted through sexual contact. Treatment and follow-up of patients with syphilis, contact tracing, treatment of infected contacts, and accurate information regarding the occupations and transmission routes of infected persons to explore high-risk groups are necessary to prevent syphilis. Additionally, to prevent syphilis, patient follow-up or epidemiological surveys should be performed in South Korea, as well as in some countries such as the US and Canada.
5. Conclusions
The high occurrence of syphilis in men and young adults found in this study regardless of their employment status, will provide basic data for developing measures to respond to syphilis in South Korea. One example of such measure is providing education and promotion for syphilis prevention in early summer in men, based on the findings regarding the seasonality of syphilis. In the future, epidemiological studies on STDs, including syphilis, should continue to be performed, and policies should be developed based on epidemiological findings.
Acknowledgments
We would like to thank Editage (www.editage.co.kr) for English language editing.
Author contributions
Conceptualization: Yeong-Jun Song, Hoyong Choi.
Data curation: Yeong-Jun Song.
Formal analysis: Yeong-Jun Song.
Methodology: Yeong-Jun Song, Hoyong Choi.
Resources: Yeong-Jun Song, Hoyong Choi.
Software: Yeong-Jun Song.
Supervision: Hoyong Choi.
Writing – original draft: Yeong-Jun Song.
Writing – review & editing: Yeong-Jun Song, Hoyong Choi.
Abbreviations:
- ARIMA
- autoregressive integrated moving average
- KCDC
- Korea Centers for Disease Control and Prevention
- STDs
- sexually transmitted diseases
The authors have no funding and conflicts of interest to disclose.
The data that support the findings of this study are available from a third party, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.
How to cite this article: Song Y-J, Choi H. Seasonality of syphilis in males through the 2011 to 2019 mandatory surveillance period: A cross-sectional study in South Korea. Medicine 2023;102:50(e36723).
References
- [1].Heymann DL, APHA, ed. Control of Communicable Diseases Manual: An Official Report of the American Public Health Association. Washington: The American Public Health Association Press; 2008. [Google Scholar]
- [2].Kenyon CR, Osbak K, Tsoumanis A. The global epidemiology of syphilis in the past century – a systematic review based on antenatal syphilis prevalence. PLoS NeglTrop Dis. 2016;10:e0004711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845–54. [DOI] [PubMed] [Google Scholar]
- [4].Clement ME, Okeke NL, Hicks CB. Treatment of syphilis: a systematic review. JAMA. 2014;312:1905–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schmidt R, Carson PJ, Jansen RJ. Resurgence of syphilis in the united states: an assessment of contributing factors. Infect Dis (Auckl). 2019;12:1178633719883282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Peterman TA, Su J, Bernstein KT, et al. Syphilis in the united states: on the rise? Expert Rev Anti Infect Ther. 2015;13:161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].World Health Organization. Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections, 2021: Accountability for the Global Health Sector Strategies 2016–2021: Actions for Impact. Geneva, Switzerland: World Health Organization; 2021. Access date February 6, 2022. Available at: https://www.who.int/publications/i/item/9789240027077 [Google Scholar]
- [9].Keuning MW, Kamp GA, Schonenberg-Meinema D, et al. Congenital syphilis, the great imitator-case report and review. Lancet Infect Dis. 2020;20:e173–9. [DOI] [PubMed] [Google Scholar]
- [10].de Oliveira SIM, de Oliveira Saraiva COP, de França DF, et al. Syphilis notifications and the triggering processes for vertical transmission: a cross-sectional study. Int J Environ Res Public Health. 2020;17:984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].World Health Organization. Global Health Sector Strategy on Sexually Transmitted Infections 2016–2021: Towards Ending STIs. Geneva, Switzerland: World Health Organization; 2016. Access date January 8, 2022. [Google Scholar]
- [12].Park S, Cho E. National infectious diseases surveillance data of South Korea. Epidemiol Health. 2014;36:e2014030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhang X, Zhang T, Pei J, et al. Time series modelling of syphilis incidence in China from 2005 to 2012. PLoS One. 2016;11:e0149401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ahmad OB, Boschi-Pinto C, Lopez AD, Murray CJ, Lozano R, Inoue M. Age Standardization of Rates: A New WHO Standard. Geneva: World Health Organization; 2001:9(10):1–4. [Google Scholar]
- [15].Bogin B, Smith BH. Evolution of the human life cycle. Am J Hum Biol. 1996;8:703–16. [DOI] [PubMed] [Google Scholar]
- [16].Harris RID. Testing for unit roots using the augmented Dickey-Fuller test. Econ Letters. 1992;38:381–6. [Google Scholar]
- [17].Billah B, King ML, Snyder RD, et al. Exponential smoothing model selection for forecasting. Int J Forecast. 2006;22:239–47. [Google Scholar]
- [18].Cleveland RB, Cleveland WS, McRae JE, et al. A seasonal-trend decomposition. J Off Stat. 1990;6:3–73. [Google Scholar]
- [19].Crawley MJ. The R Book. 2nd ed. Hoboken, NJ: John Wiley & Sons, Ltd; 2013. [Google Scholar]
- [20].Sugishita Y, Kayebeta A, Soejima K, et al. Rapid increase of syphilis in Tokyo: an analysis of infectious disease surveillance data from 2007 to 2016. Western Pac Surveill Response J. 2019;10:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ibáñez-Cervantes G, León-García G, Vargas-De-León C, et al. Epidemiological behavior and current forecast of syphilis in Mexico: increase in male population. Public Health. 2020;185:386–93. [DOI] [PubMed] [Google Scholar]
- [22].Spiteri G, Unemo M, Mårdh O, et al. The resurgence of syphilis in high-income countries in the 2000s: a focus on Europe. Epidemiol Infect. 2019;147:e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sopirala MM, Syed A, Jandarov R, et al. Impact of a change in surveillance definition on performance assessment of a catheter-associated urinary tract infection prevention program at a tertiary care medical center. Am J Infect Control. 2018;46:743–6. [DOI] [PubMed] [Google Scholar]
- [24].Choudhri Y, Miller J, Sandhu J, et al. Infectious and congenital syphilis in Canada, 2010–2015. Can Commun Dis Rep. 2018;44:43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Marcus U, Bremer V, Hamouda O. Syphilis surveillance and trends of the syphilis epidemic in Germany since the mid-90s. Euro Surveill. 2004;9:9–10. [DOI] [PubMed] [Google Scholar]
- [26].Grov C, Breslow AS, Newcomb ME, et al. Gay and bisexual men’s use of the Internet: research from the 1990s through 2013. J Sex Res. 2014;51:390–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fenton KA, Breban R, Vardavas R, et al. Infectious syphilis in high-income settings in the 21st century. Lancet Infect Dis. 2008;8:244–53. [DOI] [PubMed] [Google Scholar]
- [28].Elwood WN, Greene K. “Risks both known and unknown”: a qualitative method to assess the role of situation in HIV/STD risk and prevention. J Homosex. 2005;50:135–54. [DOI] [PubMed] [Google Scholar]
- [29].Pérez-Morente MA, Gázquez-López M, Álvarez-Serrano MA, et al. Sexually transmitted infections and associated factors in southeast Spain: a retrospective study from 2000 to 2014. Int J Environ Res Public Health. 2020;17:7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cornelisse VJ, Chow EPF, Chen MY, et al. Summer heat: a cross-sectional analysis of seasonal differences in sexual behaviour and sexually transmissible diseases in Melbourne, Australia. Sex Transm Infect. 2016;92:286–91. [DOI] [PubMed] [Google Scholar]
- [31].Wellings K, Macdowall W, Catchpole M, et al. Seasonal variations in sexual activity and their implications for sexual health promotion. J R Soc Med. 1999;92:60–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Song YJ, Cheong HK, Ki M, et al. The epidemiological influence of climatic factors on shigellosis incidence rates in Korea. Int J Environ Res Public Health. 2018;15:2209. [DOI] [PMC free article] [PubMed] [Google Scholar]