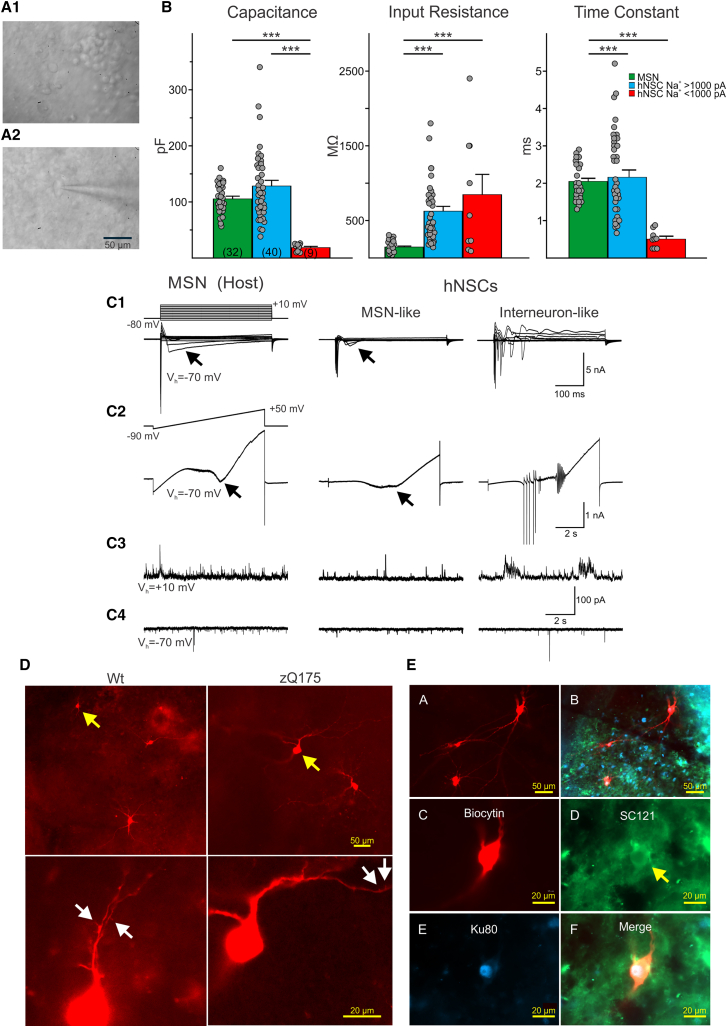
Figure 5.
Two main types of hNSCs could be distinguished based on soma size and passive and active membrane properties
(A1) IR-DIC image from transplanted hNSCs in a zQ175 mouse revealed that most cells had small, round somata. (A2) A small percentage of hNSCs had larger somata and some dendritic branches. A patch pipette attached to the cell can be seen. Example cell is from a zQ175 mouse. (B) Bar graphs show mean ± SEM of cell membrane properties of host MSNs (WT and zQ175 pooled together) compared to implanted hNSCs. Capacitance, input resistance, and time constants are shown for recorded host MSNs and mature and immature hNSCs. Statistical significance was measured using one-way ANOVA tests followed by Bonferroni post hoc tests for pairwise comparisons; ∗∗∗p < 0.001. (C1) Recordings of intrinsic currents in response to step voltage commands (10-mV steps from −80 to +10 mV) in a host MSN (left) and in an MSN-like (center) and an interneuron-like (right) hNSC. Depolarizing voltage commands induced large Na+ currents followed by inactivating Ca2+ currents (arrows) of variable amplitudes. In the interneuron-like cell, repetitive spikes were observed but no prominent Ca2+ currents. (C2) Recordings of currents in response to a ramp voltage command (8 s, from −90 to +50 mV). Some hNSCs recorded (center) had properties similar to host MSNs, both displaying Ca2+ currents (black arrows) after membrane depolarization. Other large hNSCs recorded lacked Ca2+ currents but displayed repetitive Na+ spikes (right) and were probably interneurons. (C3) Spontaneous synaptic currents recorded at +10 mV in a host MSN and in MSN-like and interneuron-like hNSCs. These currents are mostly GABAergic. (C4) Spontaneous synaptic currents recorded at −70 mV in a host MSN and in an MSN-like and an interneuron-like hNSC. These currents are most likely glutamatergic. Traces in each column are from the same cell. Calibrations on the right apply to all traces in each row. (D) Upper panels show low-magnification images of several hNSCs. Arrows (yellow) indicate two cells that displayed MSN-like electrophysiological properties. Lower panels show processes from the same cells at higher magnification. Arrows (white) indicate possible dendritic spines. (E, A) Four medium to large hNSCs were recorded within the graft. (E, B) The same image showing staining for the human markers SC121 (cytoplasmic, green) and Ku80 (nuclear, blue). (E, C) Fluorescent image of one of the large biocytin-filled hNSCs. (E, D and E) Immunostaining of the same hNSC with human stem cell markers SC121 (arrow) and Ku80. (F) Merged image showing biocytin and the two human stem cell markers. The electrophysiology of this interneuron-like hNSC is shown in (C1) and (C2), right panels. (B) Capacitance: p = 2.0007e−05 for MSN versus hNSC Na+ < 1,000 pA and p = 6.1282e−08 for hNSC Na+ > 1,000 pA versus hNSC Na+ < 1,000 pA. Input resistance: p = 5.3000e−06 for MSN versus hNSC Na+ > 1,000 pA and p = 2.6645e−05 for MSN versus hNSC Na+ < 1,000 pA. Time constant: p = 8.9566e−05 for MSN versus hNSC Na+ < 1,000 pA and p = 1.7886e−05 for hNSC Na+ > 1,000 pA versus hNSC Na+ < 1,000 pA.