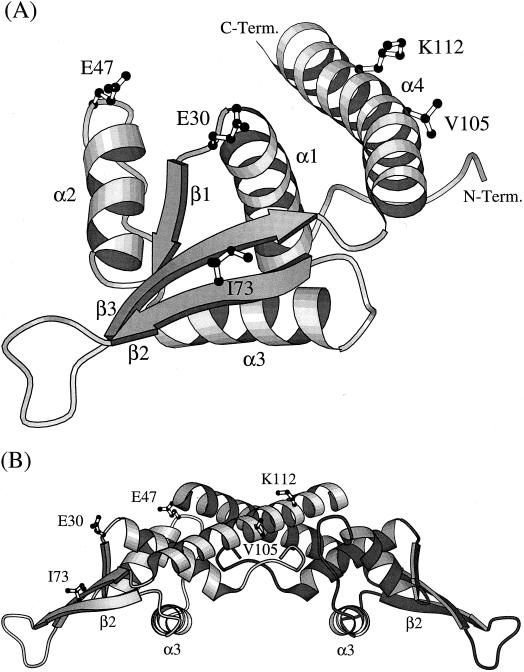
FIG. 3.
(A) A ribbon diagram showing the folded structure of an RTP monomer comprising four α helices, three β strands, and an extended loop connecting the β2 and β3 strands (5). The unstructured amino-terminal region (residues 1 to 6) is not shown. The location of the five amino acids (E30, E47, I73, V105, and K112) that are changed within four closely related Bacillus species are indicated. C-Term., C terminus; N-Term., N. terminus. (B) A ribbon diagram of a dimer of RTP showing the amino acid changes identified. The changes are indicated in only the left monomer, which is less heavily shaded. In binding to the DNA, the dimer is positioned on top of the double helix, such that its lower surface, as well as the unstructured amino-terminal region, make contact with it. The α3 helices dock into adjacent major grooves of the DNA, and the β2 strands dock into flanking minor grooves (34).