Abstract
Introduction
Fluid balance monitoring is pivotal to patients’ health. Thus, fluid balance charting is an essential part of clinical nursing documentation. This systematic review aimed to investigate and describe the quality of fluid balance monitoring in medical, surgical and intensive care units, with an emphasis on the completeness of charting data, calculation errors and accuracy, and to evaluate methods used to improve fluid balance charting.
Materials and methods
Quantitative studies involving adult patients and reporting data on fluid balance monitoring were included in the review. We searched MEDLINE, Embase, CINAHL and the Cochrane Library. The risk of bias in the included studies was assessed using tools developed by the Joanna Briggs Institute.
Results
We included a total of 23 studies, which involved 6649 participants. The studies were quasi-experimental, cohort or prevalence studies, and every third study was of low quality. Definitions of ‘completeness’ varied, as well as patient categories and time of evaluation. Eighteen studies reported the prevalence of patients with complete fluid balance charts; of those, 10 reported that not more than 50% of fluid balance charts were complete. Studies addressing calculation errors found them in 25%–35% of charts, including omissions of, for example, intravenous medications. The reported interventions consisted of various components such as policies, education, equipment, visual aids, surveillance and dissemination of results. Among studies evaluating interventions, only 38% (5 of 13) achieved compliance with at least 75% of complete fluid balance charts. Due to the heterogeneity of the studies, a meta-analysis was not possible.
Conclusion
The quality of fluid balance charting is inadequate in most studies, and calculation errors influence quality. Interventions included several components, and the impact on the completion of fluid balance charts varied.
Keywords: nurses, quality measurement, standards of care
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Fluid balance charting is a widely used tool in clinical practice but is well known for being inadequate. The low quality of fluid balance charting, as well as the prevalence of calculation errors, has been reported in studies across the world. However, a review of quality and interventions to improve it is lacking.
WHAT THIS STUDY ADDS
This review provides an overview of the quality of fluid balance charting and identifies interventions intended to improve it. We found that the quality is inadequate in medical, surgical and intensive care settings due to missing documentation or calculation errors. In addition, interventions often have not achieved sufficient improvement, some hardly any.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study indicates a need for further exploration of barriers and facilitators in fluid balance monitoring to gain knowledge to develop robust and effective interventions.
Introduction
A healthy body is in a state of fluid balance, but hospitalised patients are at risk of fluid balance disorders. Thus, fluid balance monitoring has clinical relevance to treating the patient correctly and helps determine the appropriate prescribing of fluids and diuretics essential to achieve or maintain homeostasis and healing.1 The standard fluid balance monitoring method is keeping a fluid balance chart to document the patient’s fluid input and output. Fluid balance charting is considered a fundamental nursing task and has been an essential tool in hospital practice for over 50 years.2
Fluid balance is the difference between the amount of fluid taken into the body and the amount excreted or lost. The Australian Nurses Dictionary defines it as ‘a state in which the volume of body water and its solutes (electrolytes and non-electrolytes) is within normal limits, and there is a normal distribution of fluids within the intracellular and extracellular compartments’.3
In hospitalised patients, fluid disorders are among the most common problems encountered in clinical practice4 across medical and surgical wards, and fluid balance disorders such as overhydration and dehydration can seriously affect patients’ health. Overhydration is associated with complications such as peripheral oedema and dyspnoea5 and increased mortality in patients with sepsis, cerebral haemorrhage and heart disease.6–8 Further, dehydration is associated with an increased risk of constipation, urinary tract infections and falls, prolonging hospitalisation and impairing the quality of life.9–12 Postoperative fluid balance monitoring is pivotal13 as both overhydration and dehydration can lead to complications and prolonged hospitalisation following an operation.14–16
Three main elements can assess fluid balance: clinical assessment, blood chemistry review and fluid balance charts. Clinical assessment includes vital signs, capillary refill time, tissue turgor, the amount and colour of the urine, feeling of thirst and daily weight.17 However, some of these factors have not been proven to be significantly associated with fluid balance but are used in clinical practice. Blood chemistry review may comprise creatinine and urea as well as electrolytes such as sodium and potassium.18
A fluid balance chart is a non-invasive tool that aims to keep an accurate record of a patient’s fluid status over 24 hours. The document should indicate if the patient is in fluid balance, deficit or overload.1 2 18 The input consists of fluids ingested orally, parenteral nutrition and intravenous fluids including medications (eg, antibiotics). Whether blood products should be counted in the fluid balance calculation is debatable.2 Any fluid given orally, through feeding tubes or intravenously is considered part of the fluid balance chart. The output includes all fluid losses that can be measured: urine, nasogastric drainage, vomit, liquid stool and output in drains and tubes. It differs if insensible losses from the lungs, skin and respiratory tract are included.2 17
Fluid balance charting seems relatively straightforward. Still, monitoring is often inadequate due to staff shortage and lack of time and training,1 18–20 and the charts can be challenging to interpret and calculate.21 Further, fluid volumes are estimated based on visual assessment. Studies have shown such estimations are unreliable22 23 and affected by, for example, the colour of the fluids and the shape of the container used.23 24 To clarify the scope and characteristics of the problem, a systematic overview of the literature can provide information on the quality of fluid balance in different wards and settings along with possible interventions to improve fluid balance charting.
This systematic review investigates and describes the quality of fluid balance monitoring with an emphasis on completeness, calculation errors and accuracy. The primary outcome of the review is to evaluate the completeness of fluid balance charts. Secondary outcomes include the frequency and size of calculation errors, the occurrence of missing calculations (totals) and fluid balance monitoring accuracy. Furthermore, it provides an overview of interventions used to improve fluid balance charting.
Methods
This systematic review involves quantitative studies addressing the quality of fluid balance charting in medical and surgical wards and intensive care units (ICUs).
The review is registered in the PROSPERO database of systematic reviews (registration number: CRD42021249004). Throughout the review process and in reporting the results, we worked in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.25
We did not involve patients or the public in this systematic review’s design, conduct or reporting, as it referred to specific nursing care requiring professional knowledge and insight.
Search strategy and study selection
We developed the search strategy in cooperation with an information specialist and searched the following databases—CINAHL, Embase, MEDLINE and the Cochrane Library—in November 2020 and February 2021. We repeated the search in October 2022. Additionally, we searched PROSPERO for relevant ongoing or recently completed systematic reviews and ProQuest Dissertations and Theses Global for grey literature.
The nursing environment has changed enormously during the last decades with, for example, accelerated patient pathways, implementation of electronic patient records and increased workload due to staff shortage. Thus, we restricted the searches to the publication period of 2010–2021 to evaluate contemporary practice. It included a thesaurus (eg, MeSH Terms) and free-text search, which was structured according to the PI(CO) form.26 The keywords used included “fluid balance” OR “urine output” AND “measure” OR “charting” AND “accuracy” OR “completeness” OR “quality” (search strategy as online supplemental material). Studies published in English, Danish, Norwegian and Swedish were considered for inclusion.
bmjoq-2023-002260supp001.pdf (142.3KB, pdf)
Two reviewers (LRL and ST-H) independently screened records using the software Covidence.org, which removed duplicates. First, we screened titles and abstracts based on the predetermined selection criteria and then assessed them for eligibility through full-text reading by four reviewers (LRL, MK, ST-H and NA). Reasons for the exclusion of full-text studies are provided in the PRISMA diagram. Any disagreements were resolved through discussion.
Eligibility criteria
We chose studies presenting quantitative data on fluid balance monitoring originating from fluid balance charts. Therefore, studies assessing fluid balance using invasive procedures requiring intubation or insertion of a catheter as required in measuring, for example, central venous pressure were not eligible. We excluded studies addressing fluid balance assessment only in the intraoperative phase. Only studies reporting data on total fluid balance based on input and output measurements were selected so that those exclusively reporting a single parameter (eg, urinary output) were excluded. We included studies regarding the fluid balance on a specific day as recorded in a fluid balance chart, and studies addressing the cumulative fluid balance based on fluid balance charts of several days during admission. Studies conveying fluid balance disturbances developed over time, for example, prior to admission, were only included if the study addressed fluid balance charting quality.
Research involving hospitalised patients 18 years or older and specifying the number of included patients was considered eligible. We included all study designs except case reports as long as the eligibility criteria were met. Conference abstracts were omitted.27
Quality appraisal method
Two reviewers (LRL and MK) assessed all included studies independently using quality appraisal tools developed by Joanna Briggs Institute (JBI, https://jbi.global/critical-appraisal-tools) for rigorous assessment of their methodological quality and to determine if they addressed possible bias in the design, conduct and analysis.28
Studies designed as preaudits/postaudits performed before and after an intervention targeted to improve the quality of fluid balance monitoring were defined as quasi-experimental. A prevalence study is a kind of cross-sectional study undertaken to determine the prevalence26 of, for instance, completed fluid balance charts conducted as retrospective or prospective audits. Studies were classified according to the outcome of interest; thus, for example, cohort studies could be assessed as a prevalence study if the outcome of interest was reported as a prevalence.
We rated the quality of studies as low, moderate, or high depending on the number of positive answers in the JBI instrument. The quality was rated as low if fewer than 50%, moderate if between 51% and 80%, and high if more than 80% of questions received a positive answer.29 30 We did not exclude any studies due to their low quality.
Data extraction and synthesis
Before data extraction, we developed a customised instrument inspired by a generic template in Covidence (https://www.covidence.org/) and adjusted it as necessary. Two reviewers (LRL and MK) independently extracted all data and resolved disagreements through discussion until a consensus was reached.
The data extraction included characteristics of studies (eg, first author, country, year of publication, setting, study design), participants (age, sex, reason for admission) and results on fluid balance monitoring. Completeness was defined as the proportion of complete fluid balance charts, and a complete fluid balance chart covers all intake and output and enables calculation of the 24-hour fluid balance. If applicable, we further extracted documentation of oral fluid intake, intravenous fluids, urine output, calculated totals and calculation errors. Calculation errors were defined as discrepancies between nurses’ calculations and researchers’ recalculations and comprised both erroneous mathematical calculations and incorrect calculations due to omissions of certain fluids. Furthermore, we collected data on interventions, determined as any activity or action taken with the aim of improving certain outcomes.31 We extracted the number of repeated data collections if there were multiple preinterventional or postinterventional data collections and recorded all data.
Results
Study selection
We identified 12 519 titles from screening the databases and removed 1971 duplicates. The remaining 10 548 studies were screened against the title and abstract. We included a total of 237 articles for full-text reading and assessed them for eligibility. We excluded 214 papers as they did not meet the inclusion criteria. The remaining 23 papers were included in this review. The selection process is presented in a PRISMA flow diagram25 (figure 1).
Figure 1.
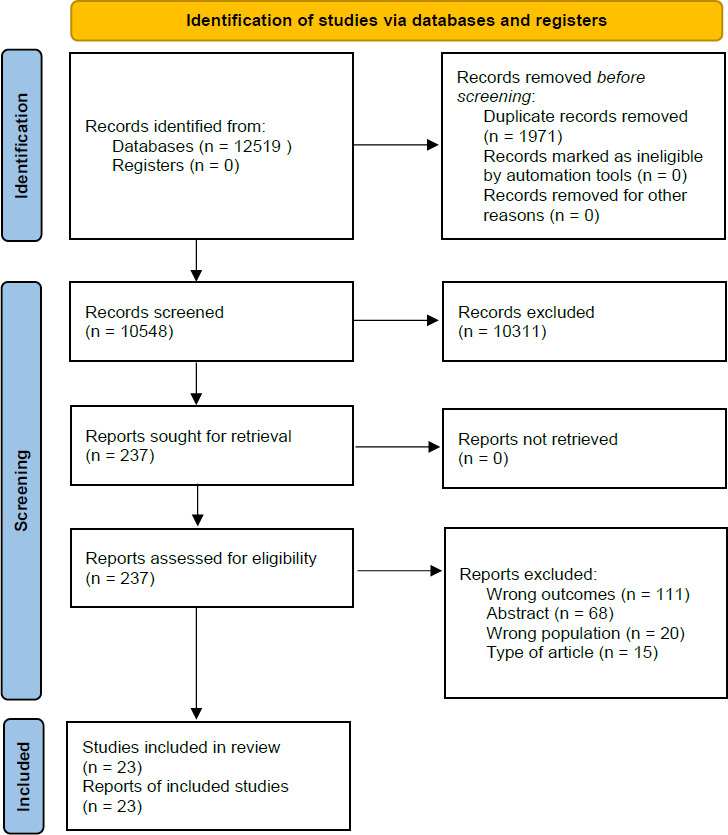
PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Characteristics of included studies
We identified 23 eligible studies published between 2010 and 2021; 10 were published between 2010 and 201432–41 and 13 between 2015 and 2021.42–54 The studies were conducted in 12 different countries on five continents; of those, 10 originated in the UK.32 36 40–43 45 47 49 54 A total of 6649 patients participated in the research, varying from 24 patients to 2199 in each study. Most studies addressed fluid balance charting on a specific day; however, two studies reported cumulative fluid balance. General characteristics, aims and findings are presented in table 1.
Table 1.
Characteristics of included studies
| Author(s), year, country | Aim | Design | Setting and participants | Sample size | Outcomes regarding fluid balance monitoring | Key findings |
| Aitken et al, (2013); UK32 | AKI Quality of care | Cohort study | Medical, surgical, AKI | 243 | Adequate FBD (%), inaccurate FB chart (%) |
51.8% of patients with AKI had adequate FBD; 43.4% of the remainder had inaccurate FB charts in case notes. |
| Alexander and Allen (2011) USA33 | Policy for FB monitoring | Quasi-experimental | Medical oncology units | 427 | Compliance with FBD | Before intervention, 12% of patients with indication of FB monitoring had FBD vs 84% after. |
| Asfour (2016); Egypt52 | Accuracy of FB monitoring | Cross-sectional | ICU except dialysis | 100 | Accuracy of calculated FB compared with researcher’s calculation | 65% of FB charts were accurate and 35% were inaccurate. |
| Baird et al, (2019); UK42 | AKI prediction score | Quasi-experimental | Orthopaedic surgery | 78 | FB monitoring (%) | Before intervention, 62% had adequate FB monitoring postoperatively vs 66% after. |
| Davies et al, (2017); UK43 | Reduce AKI alerts | Quasi-experimental | Orthopaedic trauma | 75 | Running hourly input, 6 hourly input subtotal, 6 hourly output subtotal and 24-hour total FB | Before intervention, 80% had running 1 hour totals vs 96% after; 36% had 6 hour output vs 68% after. Before intervention, 12% had 24-hour totals vs 72% after and 32% after 6 months. |
| Davies et al, (2019); Australia*44 | Relationship between calculated FB and BW | Prospective cohort study | ICU, requiring CRRT | 61 | FB charts with calculation errors (%), median deviation in millilitres | Calculation errors prevalent in 27% of charts. Median daily calculation error: 58 mL (range 1–1464 mL). Median cumulative calculation error: 131 mL (range 1–2405 mL). |
| Diacon and Bell (2014); South Africa34 |
FB monitoring and measurement accuracy | Cross-sectional | ICU | 103 | FBD (%), calculation errors (%), deviation in millilitres | 95.1% had FBD. Calculation errors: 68.9% deviated 0–500 mL, 13.5% 500–1000 mL, 6.8% 1001–2000 mL, 5.8%>2000 mL |
| Eastwood et al, (2012); Australia35 | Patients receiving IV fluids (%) | Cross-sectional | All inpatients except ICU, ED and PACU | 326 | Percentage with FB charts maintained (%) | 94% of patients receiving intravenous fluids had FB charts vs 44% receiving no intravenous fluids. |
| Herrod et al, (2010); UK36 | Prevalence of dysnatraemias and precipitating factors | Cross-sectional | Surgery Na<130 or >150 mmol/L |
55 | Complete FB charts (%) Charts with FB calculated (%), median inaccuracy in FB calculation |
28% had complete FB charts before dysnatraemia vs 44% after; 37% of charts had no FB calculation. Median calculation error: 72 mL (IQR 9–313 mL) before dysnatraemia vs 130 mL (IQR 71–400 mL) after |
| Joslin et al, (2015); UK45 | AKI recognition and management | Quasi-experimental | AKI | 192 | Complete FB chart on first day of AKI (%), days with complete FBD (%) |
Before intervention, 25% had completed FB charts on first day of AKI vs 34% (p=0.23) after. Before intervention, FBD was completed in 32% of patient days vs 45% after (p=0.002). |
| Liaw and Goh (2018); Singapore46 | Fluid intake charting | Quasi-experimental | Acute surgery | 60 | Deduction of input/output at midday and over 24 hours. Calculation of 24 hours FB. Involving patients in fluid intake documentation | Before intervention, 3.3% of the FB charts were complete (midday and 24 hours totalling) vs 100% 1 month and 6 months later. Before intervention, patients were involved in 10% of cases vs 87% 1 month later and 83% 6 months later. |
| Lim et al, (2021); Singapore53 | Ordering and documenting fluid in- and output | Cross-sectional | Acute care hospital | 2199 | Accuracy of FBD defined by recording in millilitres | Overall accuracy 77%. Oral and intravenous fluids 100% accurate, output accurate in 21% of cases |
| Madu et al, (2021); UK54 | Chart completeness and accuracy | Quasi-experimental | General medicine | 82 | Accurate measurements, calculation errors, complete documentation | Before intervention, 25% of measurements were accurate vs 39% after 1 month and 5% after 6 months, correct daily totals in 20% vs 40% after 1 month and 15% after 6 months, 14% of charts complete vs 31% after 1 month and 5% after 6 months |
| Møller et al. (2013); Denmark37 | Quality improvement | Prospective cohort study | Acute surgery, PPU |
1650 | Quality-of-care indicator: daily FBD | Before intervention, 74% had FBD vs 79% after. RR 1.07 (95% CI 1.02 to 1.13; p=0.010) |
| Perren et al, (2011); Switzerland*38 | Accuracy of FBD; agreement w BW | Cross-sectional | ICU | 147 | Complete FB charts, FB charts with calculation errors |
12% were excluded due to an incomplete FB chart; 33% of nurse-registered cumulative FB was inaccurate, errors: −3606 mL to +2020 mL. Mean absolute error of 445±668 mL |
| Pinnington et al, (2016); UK47 | Complete FBD | Quasi-experimental | Three wards | 120 | Complete FB charts | Before intervention, 32% of FB charts were completed correctly vs 92% after. In AKI patients, 20% had FBD before vs 91% after. |
| Szmuda et al, (2014); Poland39 | Calculation errors; FBD and chart completeness |
Cross-sectional | NICU and NHDU, SAH | 41 | Complete FB charts, FB miscalculations |
63.4% of FB charts were complete, 80.2% in NICU and 58.7% in NHDU (p<0.01). Fluid intake miscalculations in 27.4% of charts. Most common errors: underestimating intake (80.6%), omitting drugs (66.9%) |
| Tura et al. (2020); Ethiopia48 | Managing postpartum haemorrhage | Cross-sectional | Obstetric | 45 | Standard of care criteria: fluid intake/output chart is maintained | In 13.3%, a fluid intake/output chart was maintained. |
| Vincent and Mahendiran (2015); UK49 | Quality of FB monitoring | Quasi-experimental | General medicine | 147 | FBD, indication of FBD. Chart completion: boxes filled of all total boxes. Accurate totals=<10% error | Before intervention, 67% were on FBD (indicated in 53%) vs 38% after (indicated in 93%). Average chart completion rate before intervention was 50% vs 70% after. Average chart accuracy was 41% before vs 61% after. |
| Wakeling (2011); UK40 | FBD and FB complications; Hydrant drinking aid | Quasi-experimental | Orthopaedic, surgery, urology | 313 | Complete FB charts (%) | Before intervention, 19%–50% of FB charts were complete vs 29–62% after. Completeness improved (5–18 percentage points). |
| Walker et al. (2012); UK41 | Improve guideline adherence | Quasi-experimental | Acute wards | 101 | Completion of FB charts | Before intervention, 62.3% had FBD vs 70.8% after (p=0.36). |
| Yang et al, (2019); Taiwan51 | Compliance with FB monitoring | Quasi-experimental | Congestive heart failure | 24 | FB charts used with electrolytes and physical assessment. Patients involved in FBD | Before intervention, 58% had FB charts with physical assessment vs 100% after; 42% were involved in recording before intervention vs 75% immediately after. |
| Zhu et al, (2018); China50 | Non-pharmacological fever management | Quasi-experimental | Infectious disease, HIV | 60 | Formal assessment of fluid in- and output volume documented | Before intervention, 0% had fluid input/output documented vs 73% 10 days after intervention. |
*Studies addressing cumulative fluid balance.
AKI, acute kidney injury; BW, body weight; CRRT, continuous renal replacement therapy; ED, emergency department; FB, fluid balance; FBD, fluid balance documentation; ICU, intensive care unit; NHDU, neurosurgical high-dependency unit; NICU, neurosurgical ICU; PACU, postanaesthetic care unit; PPU, perforated peptic ulcer; SAH, subarachnoid haemorrhage.
Divergent definitions characterised studies; the words ‘complete’, ‘adequate’ and ‘accurate’ were often used interchangeably. Moreover, in the most studies, no definition was provided. Among those defining the term, there were inconsistencies in addition to disagreements on which elements were included in fluid balance calculations.2 17 A prerequisite for performing a meta-analysis is including at least two comparable studies. Due to substantial heterogeneity among studies concerning the definition of outcomes, a meta-analysis was not possible. Therefore, we performed a narrative synthesis of the findings.
Quality appraisal
The studies comprised 12 studies categorised as quasi-experimental,33 40–43 45–47 49–51 54 3 cohort studies32 37 44 and 8 prevalence studies (cross-sectional studies).34–36 38 39 48 52 53 All were appraised using the JBI tools for assessing quasi-experimental and prevalence studies. Thus, the cohort studies were evaluated using the tool for prevalence studies as the outcome of interest was presented as a prevalence.32 37 44
Only 3 studies were of high quality,37 48 53 12 of moderate quality32 34–36 38 39 43–46 50 52 and 8 of low quality.33 40–42 47 49 51 54 Details of the quality appraisal can be found in table 2. All studies assessed to be of low quality had a quasi-experimental design, explained by the higher risk of bias in quasi-experimental studies compared with prevalence studies. Reasons for a poor assessment could be missing characteristics of study participants, lack of a control group and only one pretest.
Table 2.
Quality appraisal of included studies
| JBI tool | Author(s), year | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | % | Quality appraisal |
| Quasi-experimental studies | Alexander and Allen (2011)33 | Y | N | N* | N | N | Y | Y | N | N | 44 | Low |
| Baird et al (2019)42 | Y | N | N* | N | N | Y | Y | N | N | 44 | Low | |
| Davies et al (2017)43 | Y | N | N* | N | Y | Y | Y | N | N | 56 | Moderate | |
| Joslin et al (2015)45 | Y | Y | N* | N | N | Y | Y | N | N | 56 | Moderate | |
| Liaw and Goh (2018)46 | Y | N | N* | N | N | Y | Y | N | Y | 56 | Moderate | |
| Madu et al (2021)54 | Y | N | N* | N | N | Y | Y | N | N | 44 | Low | |
| Pinnington et al (2016)47 | Y | N | N* | N | N | Y | Y | N | N | 44 | Low | |
| Vincent and Mahendiran (2015)49 | Y | N | N* | N | N | Y | Y | N | N | 44 | Low | |
| Wakeling (2011)40 | Y | N | N* | N | N | Y | Y | N | N | 44 | Low | |
| Walker et al (2012)41 | Y | N | N* | N | N | Y | Y | N | N | 44 | Low | |
| Zhu et al (2018)50 | Y | N | N* | N | N | Y | Y | Y | N | 56 | Moderate | |
| Yang et al (2019)51 | Y | N | N* | N | N | Y | Y | N | N | 44 | Low | |
| Q1: Is it clear in the study what is the cause and what is the effect? Q2: Were the participants included in any comparisons similar? Q3: Were the participants included in any comparisons receiving similar treatment/care other than the exposure or intervention of interest? *Note: ‘No’ is considered good. Q4: Was there a control group? Q5: Were there multiple measurements of the outcome both before and after the intervention/exposure? Q6: Was follow-up complete, and if not, were differences between groups in terms of their follow-up adequately described and analysed? Q7: Were the outcomes of participants included in any comparisons measured in the same way? Q8: Were outcomes measured in a reliable way? Q9: Was appropriate statistical analysis used? | ||||||||||||
| Prevalence | Aitken et al32 | Y | Y | N | Y | Y | Y | Y | N | Y | 78 | Moderate |
| Asfour52 | Y | Y | N | N | Y | Y | Y | N | Y | 67 | Moderate | |
| Davies et al (2019)44 | Y | Y | N | Y | Y | Y | Y | N | Y | 78 | Moderate | |
| Diacon and Bell34 | Y | Y | Y | N | Y | Y | Y | N | Y | 78 | Moderate | |
| Eastwood et al35 | Y | Y | N | Y | Y | Y | Y | N | Y | 78 | Moderate | |
| Herrod et al36 | Y | Y | N | Y | Y | Y | Y | N | Y | 78 | Moderate | |
| Lim et al53 | Y | Y | Y | Y | Y | Y | Y | N | Y | 89 | High | |
| Møller et al37 | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100 | High | |
| Perren et al38 | Y | Y | N | Y | N | Y | Y | N | N | 56 | Moderate | |
| Szmuda et al39 | Y | Y | N | Y | Y | Y | Y | N | Y | 78 | Moderate | |
| Tura et al48 | Y | Y | Y | Y | Y | Y | Y | N | Y | 89 | High | |
| Q1: Was the sample frame appropriate to address the target population? Q2: Were study participants sampled in an appropriate way? Q3: Was the sample size adequate? Q4: Were the study subjects and the setting described in detail? Q5: Was the data analysis conducted with sufficient coverage of the identified sample? Q6: Were valid methods used for the identification of the condition? Q7: Was the condition measured in a standard, reliable way for all participants? Q8: Was there appropriate statistical analysis? Q9: Was the response rate adequate, and if not, was the low response rate managed appropriately? | ||||||||||||
*'No' is considered good
JBI, Joanna Briggs Institute; N, no; Y, yes.
Prevalence of complete fluid balance charts
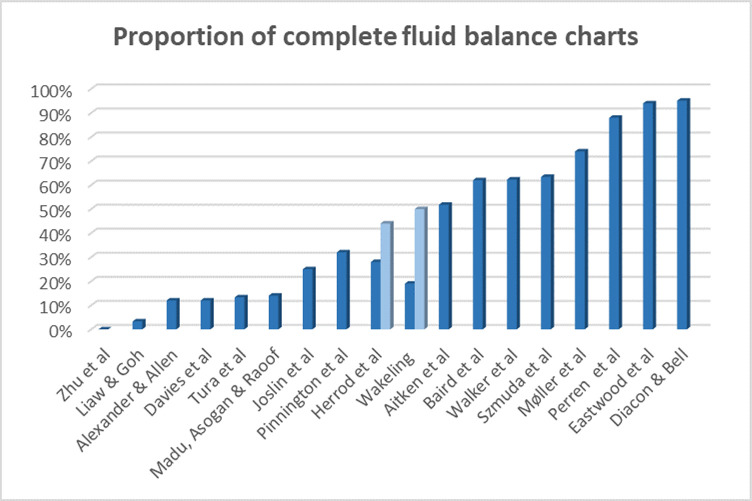
Of the included studies, 18 reported the prevalence of patients with fluid balance monitored using a fluid balance chart. Of those, seven found a proportion of complete fluid balance charts of no more than 25%,33 43 45 46 48 50 54 three studies found a proportion between 26% and 50%,36 40 47 and in five studies, the proportions were reported to be between 51% and 75%.32 37 39 41 42 Only three studies reported that more than 75% of patients had a complete fluid balance chart34 35 38 (figure 2).
Figure 2.
Overview of reported proportions of complete fluid balance charts preintervention. Two columns per study indicate that the study reported percentages from more than one ward.
Calculation errors and accuracy
Seven studies investigated the prevalence of calculation errors in fluid balance charts.34 36 38 39 44 49 54 Four were performed in ICUs.34 38 39 44 One study examining miscalculations in an ICU found a median calculation error in the daily fluid balance charts of 58 mL (range 1–1464 mL) and a cumulative median calculation error of 131 (range 1–2405 mL).44 Another study found a calculation error of more than 500 mL in 26.1% of fluid balance charts; the calculation error was between 1000 and 2000 mL in 6.8% and above 2001 mL in 5.8%.34 A third study conducted in an ICU reported inaccuracies in 33% of fluid balance charts.38 The size of the errors was between −3606 mL and +2020 mL, and the mean absolute calculation error was 445 mL±668 mL.38
A study in a neurosurgical ICU and a neurosurgical high-dependency unit reported calculation errors in 27.4% of fluid balance charts. It stated that the most frequent cause of calculation error was the underestimation of fluid intake (80.6%) primarily because of omissions of intravenous drug therapy (66.9%).39
Another study reported a median calculation error of 72 mL (IQR 9–313 mL) and 130 mL (IQR 71–400 mL) before and after the development of dysnatraemia among surgical patients; 37% did not perform a calculation of fluid balance.36 In a general medical ward, daily totals and balances were correct in only 20% of fluid charts before quality improvement initiatives.54
Moreover, an investigation of the accuracy of fluid balance charts among general medical inpatients found that the mean accuracy was 41% (<10% error was considered accurate) before initiating interventions to improve quality.49 One study defined accuracy as recorded fluid balance calculations matching the researcher’s calculated fluid balance from observation and prescription.52 Another study defined accuracy as documenting fluid in millilitres and calculated it as each recording in millilitres divided by all recordings, finding an overall accuracy of 77%. All oral and intravenous fluids were recorded correctly, but only 21% of output recordings were correct.53
Quality improvement interventions
Of the included studies, 13 describe the implementation of an intervention to improve the quality of fluid balance charts evaluated by comparing preinterventional and postinterventional audits.33 37 40–43 45–47 49–51 54 The interventions included organisational changes and adoption of policies,33 37 45 teaching and education (physical or e-learning),33 40–43 45–47 49–51 54 dialogue,41 43 46 visual aids such as posters33 43 45 47 49 54 and messages on computer background wallpapers,41 55 surveillance (eg, through monthly audits)50 51 and disseminating the results.37 41 46 51 Furthermore, several interventions incorporated some equipment such as scoring tools,42 care bundles,45 changed fluid balance charts,43 47 49 51 calculators49 54 and a drinking aid.40 Characteristics of interventions are presented in table 3.
Table 3.
Characteristics of interventions
| Author(s), year | Type of intervention | Elements in intervention | Time from implementation to evaluation |
| Alexander and Allen (2011)33 | Organisational/policy Education Visual aids |
Development of fluid balance measurement policy, computerised physician order, education of nurses and medical staff, educational poster | 2 months |
| Baird et al (2019)42 | Equipment Education Disseminating results |
Development of AKI prediction tool+intervention bundle including fluid balance monitoring, educating doctors to use the tool, presenting results at audit meetings | Immediately following each of four PDSA cycles |
| Davies et al (2017)43 | Equipment Education/dialogue Visual aids |
Redesign of fluid balance charts, posters, discussions at nursing handover, e-learning modules, informing junior doctors and encouraging close monitoring | 1 month and 7 months |
| Joslin et al (2015)45 | Organisational/policy Education Equipment Visual aids |
Hospital-wide programme to improve AKI recognition and management, AKI care bundle, educating nurses and doctors, posters on all wards, announcements on hospital intranet and screensavers | 2 years |
| Liaw and Goh (2018)46 | Equipment Education/dialogue Disseminating results |
Disseminating audit results to nurses, creating dialogue and developing strategies to overcome barriers, developing an intake chart for patients including pictorial guide, educating ward staff, providing a feedback box | 2 months and 6 months |
| Madu et al (2021)54 | Education Visual aids Equipment |
Teaching sessions, picture messages/posters, doctors prescribing fluid balance charts, weighing scales and calculators, advising staff to engage patients in recording | 4 weeks and 6 months |
| Møller et al (2013)37 | Organisational/policy Disseminating results |
Nationwide quality improvement through mandatory registration of quality-of-care indicators in the database, annual publication of results. | 2 years |
| Pinnington et al (2016)47 | Equipment Visual aids Patient education |
Implementation of a hydration assessment tool, hydration chart, fluid balance chart, urine colour chart posters and a patient information leaflet | <6 months* |
| Vincent and Mahendiran (2015)49 | Equipment Education Visual aids |
New fluid balance chart, e-learning module for nurses and HCA, posters, attendance at nursing handover, change of chart changeover (noon–noon), calculators available | <3 months* |
| Wakeling (2011)40 | Education Equipment |
Teaching sessions on hydration and fluid balance charting, implementing the Hydrant drinking aid | <4 weeks |
| Walker et al (2012)41 | Education/dialogue Visual aids Equipment Disseminating results |
Audit findings presented at meetings, key messages on computer background wallpapers, prompt on general medicine admission proforma, training of medical staff, intravenous guideline, communicating the importance of FBC at nursing handovers | 6 months |
| Yang et al (2019)51 | Equipment Education Surveillance |
Developing self-learning materials, modifying fluid balance charts, integrating into nursing information system, educating nurses and performing audits | Immediately after |
| Zhu et al (2018)50 | Education Equipment Surveillance |
Educating nurses and patients, patient leaflets, integrating into nursing information system, head nurse monitoring performance | Immediately after |
*Estimated from information in the paper.
AKI, acute kidney injury; FBC, fluid balance chart; HCA, Healthcare assistant; PDSA, Plan-Do-Study-Act.
The effect of the implemented interventions varied, and so did the time from intervention to evaluation. In five studies, the researchers achieved an improvement, indicating that at least 75% of fluid balance charts were complete and correctly filled after the intervention.33 37 46 47 51 In another five studies, the final result was within the interval of 50%–75%.40–42 49 50 The quality improved by 4–20 percentage points in four of the latter, but a single study reported an improvement from 0% to 73%.50 Three studies found that less than 50% of fluid balance charts were completed and correct after an intervention.43 45 54 A final study found an immediate quality improvement (72% complete fluid balance charts); however, after 6 months, the quality decreased to 32%.43
Discussion
This systematic review had three major findings. First, we found that although fluid balance charting is common practice in medical, surgical and ICUs, the quality of fluid balance charting is inadequate. Second, calculation errors are also common. Third, all interventions included at least two components, but the time of evaluation and the impact on the completion of fluid balance charts varied.
Quality of fluid balance charting
Half of the included studies reported that less than 50% of the fluid balance charts were complete and correctly filled,33 36 40 43 45–48 50 54 indicating that insufficient fluid balance documentation is a considerable challenge. Fluid balance charts guide clinical decisions, including prescription of intravenous fluid or medication and interventions to ensure appropriate care and reduce the risk of complications and fluid balance disorders. Thus, a thoroughly kept fluid balance chart contributes valuable data. On the contrary, it can be counterproductive if not adequately completed and put patient safety at risk by leading to erroneous conclusions.19 20 56
A compelling question related to the quality of fluid balance monitoring is what is meant by ‘complete’ fluid balance charts. A fluid balance chart may seem complete even though some documentation is missing, indicating that certainty regarding the completeness of charts can only be determined through observations. Divergent definitions or no definition at all complicate the comparison of results. The variety of definitions may, thus, express a lack of shared understanding of fluid balance monitoring. Studies show that a standardised nursing language can improve communication among healthcare professionals, adherence to standards of care and quality of care.57 58 Therefore, a shared definition of complete fluid balance monitoring may improve charting accuracy and would enable comparisons across settings.
Calculation errors
The second major finding was that erroneous calculation of fluid balance was a common and significant problem, with calculation errors in 25%–35% of the fluid balance charts.34 38 39 44 Further, erroneous daily fluid balance chartings lead to increased cumulative errors44 with a range of several litres.38 Naturally, the size of calculation errors determines whether they are of clinical significance in a specific patient category. Thus, it may be of greater interest to determine how many had a calculation error deviating, for example, more than 500 mL as this may be clinically relevant. One study reports that 26.1% had a calculation error of more than 500 mL, and half of those exceeded 1000 mL.34 However, establishing the clinically relevant accuracy threshold is difficult as it varies based on patient variables like diagnosis and age. Further, as the severity of the illness and comorbidities of patients rise, the vulnerability towards fluid balance disturbances increases, and the margin of error is reduced.59 Anyhow, this review demonstrates the necessity of improving fluid balance charting accuracy to ensure the charts’ credibility and utility.
According to several authors, the cause of errors was the manually calculated fluid balance.34 39 44 However, calculation errors can be conceptual, arithmetical or computational60 and may occur due to interruptions and time pressure.61 Ensuring access to pocket calculators44 49 or applying electronic patient records automatically calculating fluid balance based on documented information39 may minimise computational errors. A study evaluated the effect of a clinical information system and found that it saved time, for instance, due to automatic fluid balance calculation. Furthermore, staff positively evaluated the electronic record as it improved charting quality.62 Another study reported that most nurses (75%) believed electronic health records improved nursing documentation.63
Another cause of errors was a lack of documentation, such as omitting intravenous medication.39 Omissions in nursing care are recognised as a comprehensive challenge related to the shortage of nurses and high patient-to-nurse ratios.64 A qualitative study exploring regularly missed nursing care highlighted fluid balance monitoring as an essential theme.55 Reasons for this lack may include staff shortage, inappropriate use of staff resources and ineffective delegation.55
Additional challenges are an inaccurate estimation of oral fluid volumes and potential typing errors if data are entered manually.44 It is possible that a higher degree of automation can prevent these types of inaccuracies.
Interventions
The third major theme in this review was to evaluate interventions developed to improve the quality of fluid balance monitoring. Across studies, multiple components were identified as tools to improve fluid balance charting. All interventions involve several interacting components, and most target different groups or behaviours; hence, all analysed interventions can be characterised as complex. The advantage of an intervention containing several elements is that it may address various challenges simultaneously, thus increasing the probability of success.65 On the other hand, interventions perceived as simple are more easily evaluated and implemented.66 Therefore, an effective intervention should include all parameters in fluid balance charting as simply as possible.
All interventions except one involve education offered to doctors, nursing staff, or patients, but the impact varies. Possible reasons for this are the information’s relevance, delivery and whether all stakeholders received this education. Interestingly, four of the five most effective interventions include some patient involvement either by involving patients in recording fluids46 51 or informing patients through tailored education or leaflets.47 50 This indicates that involving patients in their care during hospitalisation may be beneficial. Two systematic reviews found that involving patients with chronic diseases in self-monitoring motivates them to manage their condition67 and improves outcomes such as readmission rates.68
In addition, the form of delivery may affect the results (eg, whether teaching was delivered to staff on all shifts, the duration of teaching). However, these details were only sporadically described. A review addressing electronic health record education found that training should be interactive and based on daily routines and nursing workflow.69 This may also apply to fluid balance monitoring education, but studies are needed to identify effective learning strategies to enhance the quality.
Moreover, integrating equipment (eg, care bundles or visual aids such as posters) is widely used in both effective interventions and those with hardly any effect, making it difficult to determine whether these are useful solutions. A review examining barriers and facilitators in implementing care bundles found that the number and complexity of elements affected compliance. Fewer elements and low complexity were associated with increased compliance,70 as were evaluative and iterative implementation strategies (eg, performing audits and developing stakeholder relationships). Furthermore, providing feedback was more effective than reminders such as posters and screen savers.70
Another tool is electronic patient records, which are integrated into nursing practice in many clinical settings. Taking advantage of the opportunities of electronic patient records, such as computerised physician orders,33 electronic reminders, and integrating fluid balance documentation50 51 and fluid balance calculation,56 may improve fluid balance charting.
Hence, automating fluid balance charting by using electronic patient records combined with equipment developed to automatically measure fluid intake and output may enhance charting quality. However, understanding the barriers and enablers in fluid balance charting is necessary to create effective solutions.
Other factors may affect the effectiveness of an intervention (eg, the intervention’s extensiveness, whether the components are well chosen and how they are interrelated). The implementation strategy itself is of utmost importance, addressing resistance towards the intervention and increasing acceptance.71 However, most included studies describe these aspects superficially or not at all.
A final factor that may influence the observed effects of interventions is the time of evaluation, which varied among studies from immediately to 2 years after implementation. The timing of the evaluation can have a significant impact, as shown in one study that found an immediate improvement from 12% to 72%; however, compliance fell to 32% after 6 months,43 indicating that a short-term improvement may not lead to long-term behaviour change. This phenomenon is described as a ‘honeymoon period’, and researchers should be cautious when interpreting effects less than 6 months from implementation.72 Among the most effective interventions (≥75% completed fluid balance charts), two were evaluated 6 months or more after implementation,37 46 whereas the three others were assessed after less than 233 51 and 6 months.47
Recommendations
Calculation errors pointed to in this review may be prevented by using electronic patient records, where fluid balance calculations are performed automatically and are no longer based on human calculation.39 44 62 By exclusively using fluid containers with measuring lines or through automated measuring inaccuracies related to estimations can be avoided.22 23 Additionally, interactive teaching based on daily practice for all stakeholders54 69 and involving and motivating patients to self-monitor may enhance quality.46 67 Care bundles should have few components, be straightforward, and of low complexity.66 70 Continuous attention to fluid balance charting (eg, through disseminating audit results) is required to achieve and maintain improvement.70
Limitations
This systematic review had several limitations. To begin, we conducted a broad search for literature, including only published papers. Due to the widespread problem of fluid balance charting in clinical practice, we suspect much information is available only for internal use. Thus, this review represents the quality of fluid balance monitoring generated by a systematic method but not necessarily a complete overview. Furthermore, we limited our search to the time frame of 2010 to the present, thus excluding older literature. The rationale for this decision was that the main objective of the review was to evaluate recent quality, but by analysing previous studies, we may have obtained different knowledge.
Other limitations relate to the studies included, the quality of which varied. Every third study was of low quality; thus, the power of the conclusions drawn based on them is limited. Nevertheless, we did not exclude low-quality studies as we chose not to risk omitting research from daily practice. Second, the studies are characterised by significant heterogeneity in defining outcomes, and the patients included are not comparable. Finally, the timing of the evaluation of interventions differed, making comparisons across studies difficult.
Conclusion
In conclusion, the quality of fluid balance monitoring varies, but most studies report it as inadequate, influenced by calculation errors. Implemented interventions designed to improve the quality of fluid balance monitoring had varying impacts, and in most studies, the effect was unsubstantial. Furthermore, a short-term improvement may not lead to long-term behaviour change.
Therefore, there is a need for in-depth qualitative knowledge to understand nurses’ attitudes towards and opinions of fluid balance monitoring and the perceived barriers. Further, increased knowledge of the patients’ perspective may be beneficial. Based on this understanding, innovative and robust fluid balance monitoring methods must be developed.
Footnotes
Contributors: LRL developing study design and search strategy, screening abstracts and titles, full text reading, data extraction, analysis, writing first draft of manuscript. MK full text reading, data extraction, analysis, revising manuscript. ST-H screening abstracts and titles, revising manuscript. IG developing study design, revising manuscript. AOB developing study design, revising manuscript. NA developing study design, full text reading, revising manuscript. NA is the guarantor of this study.
Funding: Interreg Öresund-Kattegat-Skagerrak, grant number: NYPS 20341110.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Holroyd S. Frequency volume charts and fluid balance monitoring: getting it right. J Commun Nurs 2020;34:4. [Google Scholar]
- 2.McGloin S. The INS and outs of fluid balance in the acutely ill patient. Br J Nurs 2015;24:14–8. 10.12968/bjon.2015.24.1.14 [DOI] [PubMed] [Google Scholar]
- 3.Australian Nurses’ Dictionary . Fluid balance (7th edn). Elsevier, 2020. [Google Scholar]
- 4.Verbalis JG. Disorders of Water Balance. Brenner and Rector’s The Kidney (11th edn). Elsevier, 2019. [Google Scholar]
- 5.Albert NM. Fluid management strategies in heart failure. Crit Care Nurse 2012;32:20–32; 10.4037/ccn2012877 [DOI] [PubMed] [Google Scholar]
- 6.Sakr Y, Rubatto Birri PN, Kotfis K, et al. Higher fluid balance increases the risk of death from sepsis: results from a large International audit. Crit Care Med 2017;45:386–94. 10.1097/CCM.0000000000002189 [DOI] [PubMed] [Google Scholar]
- 7.Shen Y, Huang X, Hu Y, et al. Positive fluid balance is associated with increased in-hospital mortality in patients with intracerebral hemorrhage. Brain Inj 2019;33:212–7. 10.1080/02699052.2018.1539870 [DOI] [PubMed] [Google Scholar]
- 8.Trejnowska E, Skoczyński S, Armatowicz P, et al. The importance of fluid balance in critically ill patients: a retrospective observational study. Kardiol Pol 2019;77:1147–54. 10.33963/KP.14991 [DOI] [PubMed] [Google Scholar]
- 9.El-Sharkawy AM, Sahota O, Lobo DN. Acute and chronic effects of hydration status on health. Nutr Rev 2015;73:97–109. 10.1093/nutrit/nuv038 [DOI] [PubMed] [Google Scholar]
- 10.Wilson DL. Hydration and Older People in the UK Addressing the Problem, Understanding the Solutions. Parliamentary Hydration Forum, 2014. [Google Scholar]
- 11.Chen C-L, Liang T-M, Chen H-H, et al. Constipation and its associated factors among patients with dementia. Int J Environ Res Public Health 2020;17:9006. 10.3390/ijerph17239006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamrick I, Norton D, Birstler J, et al. Association between dehydration and falls. Mayo Clin Proc Innov Qual Outcomes 2020;4:259–65. 10.1016/j.mayocpiqo.2020.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helander EM, Webb MP, Menard B, et al. Metabolic and the surgical stress response considerations to improve postoperative recovery. Curr Pain Headache Rep 2019;23:33. 10.1007/s11916-019-0770-4 [DOI] [PubMed] [Google Scholar]
- 14.Brandstrup B, Tønnesen H, Beier-Holgersen R, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg 2003;238:641–8. 10.1097/01.sla.0000094387.50865.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan HYL, Cheng A, Cheung SSS, et al. Association between dehydration on admission and postoperative complications in older persons undergoing Orthopaedic surgery. J Clin Nurs 2018;27:3679–86. 10.1111/jocn.14336 [DOI] [PubMed] [Google Scholar]
- 16.Wuethrich PY, Burkhard FC, Thalmann GN, et al. Restrictive deferred hydration combined with preemptive norepinephrine infusion during radical cystectomy reduces postoperative complications and hospitalization time: a randomized clinical trial. Anesthesiology 2014;120:365–77. 10.1097/ALN.0b013e3182a44440 [DOI] [PubMed] [Google Scholar]
- 17.Scales K, Pilsworth J. The importance of fluid balance in clinical practice. Nursing Standard 2008;22:50–7. 10.7748/ns2008.07.22.47.50.c6634 [DOI] [PubMed] [Google Scholar]
- 18.Shepherd A. Measuring and managing fluid balance. Nurs Times 2011;107:12–6. [PubMed] [Google Scholar]
- 19.Chung LH, Chong S, French P. The efficiency of fluid balance charting: an evidence-based management project. J Nurs Manag 2002;10:103–13. 10.1046/j.0966-0429.2001.00296.x [DOI] [PubMed] [Google Scholar]
- 20.Georgiades D. A balancing act: maintaining accurate fluid balance charting. Clinical Update 2016:5. [PubMed] [Google Scholar]
- 21.Tang VCY, Lee EWY. Fluid balance chart: do we understand it Clinical Risk 2010;16:10–3. 10.1258/cr.2009.090005 [DOI] [Google Scholar]
- 22.Tattersall C. Nursing staff’s ability to gauge fluid intake. Nurs Times 2016;112:19–22. 10.1007/s41193-016-0199-7 [DOI] [Google Scholar]
- 23.Michelsen CF, Svendsen MBS, Bagger ML, et al. A study on accuracy and precision of fluid volume measurements by nurses, patients and healthy persons in a clinical setting. Nurs Open 2022;9:1303–10. 10.1002/nop2.1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Troy DM, Attwood AS, Maynard OM, et al. Effect of glass shape on the pouring accuracy of liquid volume. PLoS One 2018;13:e0204562. 10.1371/journal.pone.0204562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polit DF, Beck CT. Nursing Research. Generating and Assessing Evidence for Nursing Practice (10th edn). Wolters Kluwer, 2017. [Google Scholar]
- 27.Harris JD, Quatman CE, Manring MM, et al. How to write a systematic review. Am J Sports Med 2014;42:2761–8. 10.1177/0363546513497567 [DOI] [PubMed] [Google Scholar]
- 28.Aromataris E MZE . JBI Manual for Evidence Synthesis. Joanna Briggs Institute, 2020. [Google Scholar]
- 29.Benenson I, Waldron FA, Jadotte YT, et al. Risk factors for hypertensive crisis in adult patients: a systematic review. JBI Evid Synth 2021;19:1292–327. 10.11124/JBIES-20-00243 [DOI] [PubMed] [Google Scholar]
- 30.Cicolo EA, Nishi FA, Peres HHC, et al. Effectiveness of the Manchester triage system on time to treatment in the emergency Department: a systematic review. JBI Evid Synth 2020;18:56–73. 10.11124/JBISRIR-2017-003825 [DOI] [PubMed] [Google Scholar]
- 31.Richards DA. The complex interventions framework. In: Richards DAH, Rahm I, eds. Complex interventions in health An overview of research methods. Routledge, 2015. 10.4324/9780203794982 [DOI] [Google Scholar]
- 32.Aitken E, Carruthers C, Gall L, et al. Acute kidney injury: outcomes and quality of care. QJM 2013;106:323–32. 10.1093/qjmed/hcs237 [DOI] [PubMed] [Google Scholar]
- 33.Alexander L, Allen D. Establishing an evidence-based inpatient medical oncology fluid balance measurement policy. Clin J Oncol Nurs 2011;15:23–5. 10.1188/11.CJON.23-25 [DOI] [PubMed] [Google Scholar]
- 34.Diacon A, Bell J. Investigating the recording and accuracy of fluid balance monitoring in critically ill patients. S Afr J Crit Care 2014;30:55. 10.7196/SAJCC.193 [DOI] [Google Scholar]
- 35.Eastwood GM, Peck L, Young H, et al. Intravenous fluid administration and monitoring for adult ward patients in a teaching hospital. Nurs Health Sci 2012;14:265–71. 10.1111/j.1442-2018.2012.00689.x [DOI] [PubMed] [Google Scholar]
- 36.Herrod PJJ, Awad S, Redfern A, et al. Hypo- and Hypernatraemia in surgical patients: is there room for improvement World J Surg 2010;34:495–9. 10.1007/s00268-009-0374-y [DOI] [PubMed] [Google Scholar]
- 37.Møller MH, Larsson HJ, Rosenstock S, et al. Quality-of-care initiative in patients treated surgically for perforated peptic ulcer. Br J Surg 2013;100:543–52. 10.1002/bjs.9028 [DOI] [PubMed] [Google Scholar]
- 38.Perren A, Markmann M, Merlani G, et al. Fluid balance in critically ill patients. should we really rely on it? Minerva Anestesiol 2011;77:802–11. [PubMed] [Google Scholar]
- 39.Szmuda T, Waszak PM, Rydz C, et al. The challenges of Hypervolemic therapy in patients after subarachnoid haemorrhage. Neurol Neurochir Pol 2014;48:328–36. 10.1016/j.pjnns.2014.09.001 [DOI] [PubMed] [Google Scholar]
- 40.Wakeling J. Improving the hydration of hospital patients. Nurs Times 2011;107:21–3. [PubMed] [Google Scholar]
- 41.Walker GE, Stewart-Parker E, Chinthapalli S, et al. Intravenous fluid use in the acutely unwell adult medical inpatient: improving practice through a clinical audit process. J R Coll Physicians Edinb 2012;42:211–5. 10.4997/JRCPE.2012.304 [DOI] [PubMed] [Google Scholar]
- 42.Baird DP, Rae F, Beecroft C, et al. Introducing an AKI predictive tool for patients undergoing Orthopaedic surgery. BMJ Open Qual 2019;8:e000306. 10.1136/bmjoq-2017-000306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davies A, Srivastava S, Seligman W, et al. Prevention of acute kidney injury through accurate fluid balance monitoring. BMJ Open Qual 2017;6:e000006. 10.1136/bmjoq-2017-000006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davies H, Leslie GD, Morgan D, et al. A comparison of compliance in the estimation of body fluid status using daily fluid balance charting and body weight changes during continuous renal replacement therapy. Aust Crit Care 2019;32:83–9. 10.1016/j.aucc.2017.12.090 [DOI] [PubMed] [Google Scholar]
- 45.Joslin J, Wilson H, Zubli D, et al. Recognition and management of acute kidney injury in hospitalised patients can be partially improved with the use of a care bundle. Clin Med 2015;15:431–6. 10.7861/clinmedicine.15-5-431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liaw YQ, Goh ML. Improving the accuracy of fluid intake charting through patient involvement in an adult surgical ward: a best practice implementation project. JBI Database System Rev Implement Rep 2018;16:1709–19. 10.11124/JBISRIR-2017-003683 [DOI] [PubMed] [Google Scholar]
- 47.Pinnington S, Ingleby S, Hanumapura P, et al. Assessing and documenting fluid balance. Nurs Stand 2016;31:46–54. 10.7748/ns.2016.e10432 [DOI] [PubMed] [Google Scholar]
- 48.Tura AK, Aboul-Ela Y, Fage SG, et al. Introduction of criterion-based audit of postpartum hemorrhage in a University hospital in Eastern Ethiopia: implementation and considerations. Int J Environ Res Public Health 2020;17:9281. 10.3390/ijerph17249281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vincent M, Mahendiran T. Improvement of fluid balance monitoring through education and rationalisation. BMJ Qual Improv Rep 2015;4:u209885.w4087. 10.1136/bmjquality.u209885.w4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu Z, Yang F, Wang L, et al. Non-pharmacological fever management for persons living with HIV: a best practice implementation project. JBI Database System Rev Implement Rep 2018;16:791–801. 10.11124/JBISRIR-2017-003347 [DOI] [PubMed] [Google Scholar]
- 51.Yang S-H, Mu P-F, Wu H-L, et al. Fluid balance monitoring in congestive heart failure patients in hospital: a best practice implementation project. JBI Database System Rev Implement Rep 2019;17:2202–11. 10.11124/JBISRIR-2017-004021 [DOI] [PubMed] [Google Scholar]
- 52.Asfour HI. Fluid balance monitoring accuracy in intensive care units. IOSR 2016;05:53–62. 10.9790/1959-0504015362 Available: http://iosrjournals.org/iosr-jnhs/pages/5(4)Version-1.html [DOI] [Google Scholar]
- 53.Lim SH, Lim ML, Aloweni FAB, et al. Audit of the appropriateness and accuracy of fluid intake and output monitoring: experience in a tertiary hospital. Br J Nurs 2021;30:660–4. 10.12968/bjon.2021.30.11.660 [DOI] [PubMed] [Google Scholar]
- 54.Madu A, Asogan H, Raoof A. Education and training as key drivers for improving the quality of fluid balance charts: findings from a quality improvement project. BMJ Open Qual 2021;10:e001137. 10.1136/bmjoq-2020-001137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalisch BJ. Missed nursing care: a qualitative study. J Nurs Care Qual 2006;21:306–13; 10.1097/00001786-200610000-00006 [DOI] [PubMed] [Google Scholar]
- 56.Chavin G, Chow G. Maintaining proper fluid balance in the postoperative urologic patient. Contemp Urol 2008;20:30–5. [Google Scholar]
- 57.Fennelly O, Grogan L, Reed A, et al. Use of standardized Terminologies in clinical practice: A Scoping review. Int J Med Inform 2021;149:S1386-5056(21)00057-5. 10.1016/j.ijmedinf.2021.104431 [DOI] [PubMed] [Google Scholar]
- 58.Rutherford M. Standardized nursing language: what does it mean for nursing practice Online J Issues Nurs 2008;13:1–9. 10.3912/OJIN.Vol13No01PPT05 [DOI] [Google Scholar]
- 59.McGee WT, Raghunathan K. Physiologic goal-directed therapy in the perioperative period: the volume prescription for high-risk patients. J Cardiothorac Vasc Anesth 2013;27:1079–86. 10.1053/j.jvca.2013.04.019 [DOI] [PubMed] [Google Scholar]
- 60.Eastwood KJ, Boyle MJ, Williams B, et al. Numeracy skills of nursing students. Nurse Educ Today 2011;31:815–8. 10.1016/j.nedt.2010.12.014 [DOI] [PubMed] [Google Scholar]
- 61.Colligan L, Bass EJ. Interruption handling strategies during Paediatric medication administration. BMJ Qual Saf 2012;21:912–7. 10.1136/bmjqs-2011-000292 [DOI] [PubMed] [Google Scholar]
- 62.Donati A, Gabbanelli V, Pantanetti S, et al. The impact of a clinical information system in an intensive care unit. J Clin Monit Comput 2008;22:31–6. 10.1007/s10877-007-9104-x [DOI] [PubMed] [Google Scholar]
- 63.Moody LE, Slocumb E, Berg B, et al. Electronic health records documentation in nursing. nurses' perceptions, attitudes, and preferences. Comput Inform Nurs 2004;22:337–44. 10.1097/00024665-200411000-00009 [DOI] [PubMed] [Google Scholar]
- 64.Griffiths P, Recio-Saucedo A, Dall’Ora C, et al. The association between nurse staffing and omissions in nursing care: A systematic review. J Adv Nurs 2018;74:1474–87. 10.1111/jan.13564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Skivington K, Matthews L, Simpson SA, et al. A new framework for developing and evaluating complex interventions: update of medical research Council guidance. BMJ 2021;374:n2061. 10.1136/bmj.n2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Greenhalgh T, Robert G, Macfarlane F, et al. Diffusion of innovations in service organizations systematic review and recommendations. Milbank Q 2004;82:581–629. 10.1111/j.0887-378X.2004.00325.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morton K, Dennison L, May C, et al. Using Digital interventions for self-management of chronic physical health conditions: A meta-Ethnography review of published studies. Patient Educ Couns 2017;100:616–35. 10.1016/j.pec.2016.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McBain H, Shipley M, Newman S. The impact of self-monitoring in chronic illness on Healthcare utilisation: a systematic review of reviews. BMC Health Serv Res 2015;15:565. 10.1186/s12913-015-1221-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ting J, Garnett A, Donelle L. Nursing education and training on electronic health record systems: an integrative review. Nurse Educ Pract 2021;55:S1471-5953(21)00204-3. 10.1016/j.nepr.2021.103168 [DOI] [PubMed] [Google Scholar]
- 70.Gilhooly D, Green SA, McCann C, et al. Barriers and Facilitators to the successful development, implementation and evaluation of care bundles in acute care in hospital: a Scoping review. Implement Sci 2019;14:47. 10.1186/s13012-019-0894-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sermeus W. Modelling process and outcomes. In: Richards DAH, Rahm I, eds. Complex interventions in health An overview of research methods. Routledge, 2015. [Google Scholar]
- 72.Tucker P. Compressed working weeks. ILO, 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjoq-2023-002260supp001.pdf (142.3KB, pdf)
Data Availability Statement
No data are available.