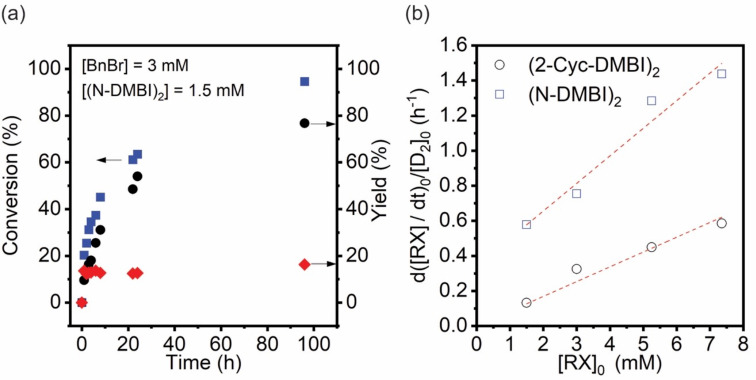
Figure 2.
(a) A representative temporal evolution of % conversion (blue squares), % toluene yield (red diamonds), and % bibenzyl yield (black circles) during the dark dehalogenation reaction of benzyl bromide (BnBr) using (N-DMBI)2 in THF (these data were acquired using 3 mM BnBr and 1.5 mM of (N-DMBI)2. (b) Plot of [D2]0-normalized initial reaction rate (d([RX]/dt)0/[D2]0) as a function of the initial benzyl bromide concentration ([RX]0) obtained from several experiments of the type shown in part (a) for different [D2] and [RX] for both D2 = (Cyc-DMBI)2 and (N-DMBI)2. For these slow reactions, the “initial” rates were estimated from the change of substrate concentration over the first 30 min reaction time.