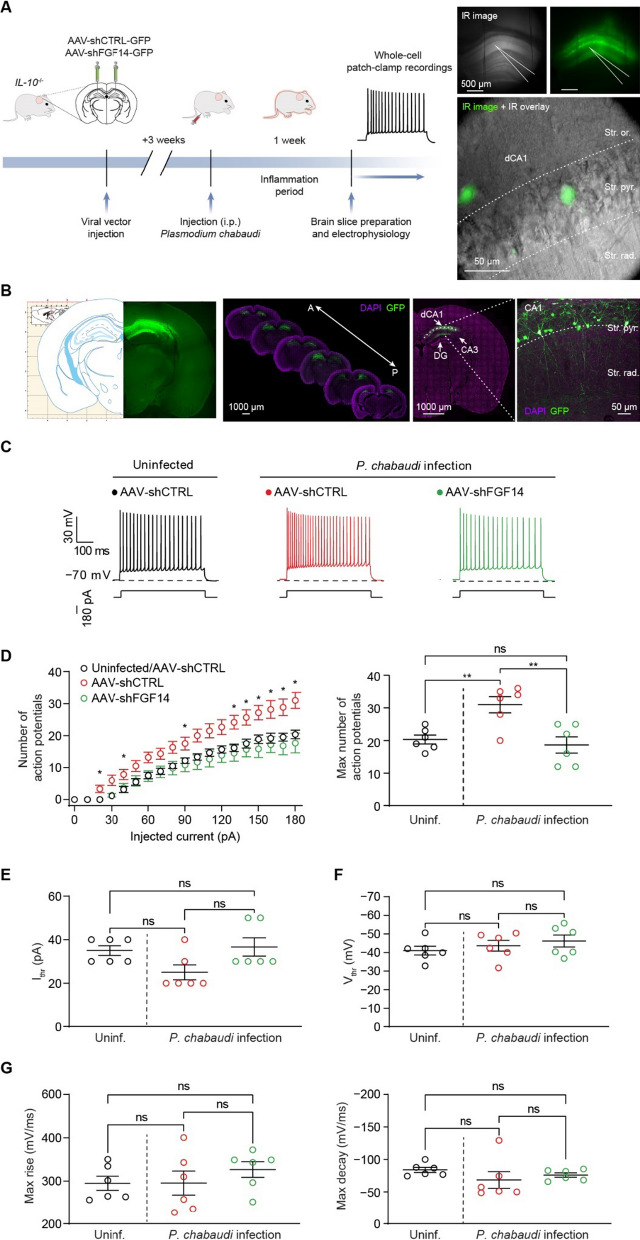
Fig. 4.
In vivo genetic silencing of FGF14 mitigates hyperexcitability of CA1 neurons caused by eCM. A Left: timeline for stereotaxic surgery, P. chabaudi infection, and electrophysiological recordings. Right: representation of whole-cell patch-clamp recording in GFP.+ CA1 pyramidal neuron. B Confirmation of accurate vector placement in CA1 region of hippocampus. C Representative traces of action potentials fired by CA1 neurons in slices from the indicated experimental groups at the 180-pA injected current step. D Left: number of action potentials fired by CA1 neurons over a range of injected current stimuli. Right: comparison of the maximum number of action potentials fired by CA1 neurons among the indicated experimental groups. E, F Comparison of the current threshold (Ithr; E) and voltage threshold (Vthr; F) for CA1 neuron action potential initiation among the indicated experimental groups. G Comparison of action potential kinetics among the indicated experimental groups. Data are mean ± SEM (n = 6 neurons/group; slices from N = 3 mice per group). Statistical significance was assessed using a one-way ANOVA with post hoc Tukey’s multiple comparisons test. In (D, right–G): ns not significant; **p < 0.01. In (D, left), * denotes current steps at which the number of action potentials fired by CA1 neurons from infected mice injected with AAV-shCTRL is significantly greater (p is at least < 0.05) than the number of action potentials fired by CA1 neurons from uninfected mice injected with AAV-shCTRL and infected mice injected with AAV-shFGF14