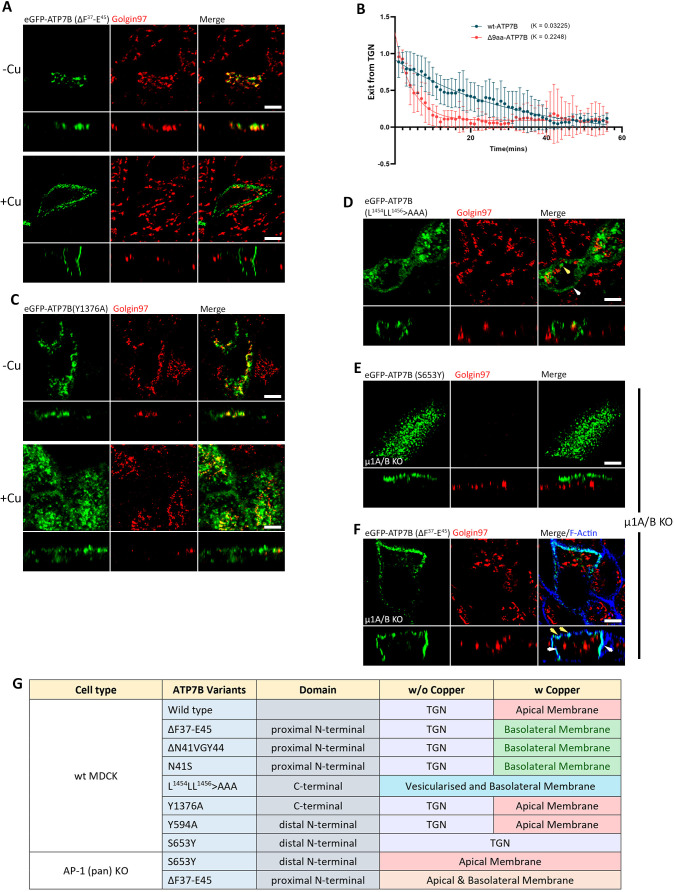
Fig. 7.
ATP7B mutants reveal additional regulation for apical polarity. (A) Images showing the 9-amino-acid (aa) deletion mutant (ΔF37-E45-ATP7B; Δ9aa) trafficking to basolateral surface in copper-treated conditions. (B) Fitted curve of decreased pixel count of eGFP–ATP7B and Δ9aa mutant in copper-treated conditions, showing dispersion of Cu-ATPases and their exit rate. The higher k-value of the Δ9aa mutant shows a faster Golgi exit rate than the wild-type (wt) ATP7B (N for ATP7B: 13, Δ9aa mutant: 6). Results are mean±s.d. (C) Confocal image of Y1376A mutant of ATP7B showing wild-type-like phenotype. (D) Image showing that the tri-leucine mutant of ATP7B (i.e. L1454LL1456>AAA) has lost its TGN localization and localizes to the basolateral surface (white arrowhead) as well as in endosomes (yellow arrowhead). (E) Image of S653Y (a non-Golgi exiting mutant of ATP7B) in AP-1 KO cells (i.e. µ1A/B double knockout cells), which has a constitutive localization on the apical surface of the plasma membrane. (F) Confocal image of ΔF37-E45 (a basolateral-targeting mutant) in AP-1 (pan) KO cells showing loss of TGN retention as well as in its polarity because of constitutive localization to both the surfaces of the plasma membrane (apical surface marked by yellow arrowhead and basolateral surface marked by white arrowhead). Images representative of three repeats. Scale bars: 5 µm. (G) Tabulated summary of trafficking phenotypes of ATP7B variants in wild-type and AP-1 KO MDCK cells.