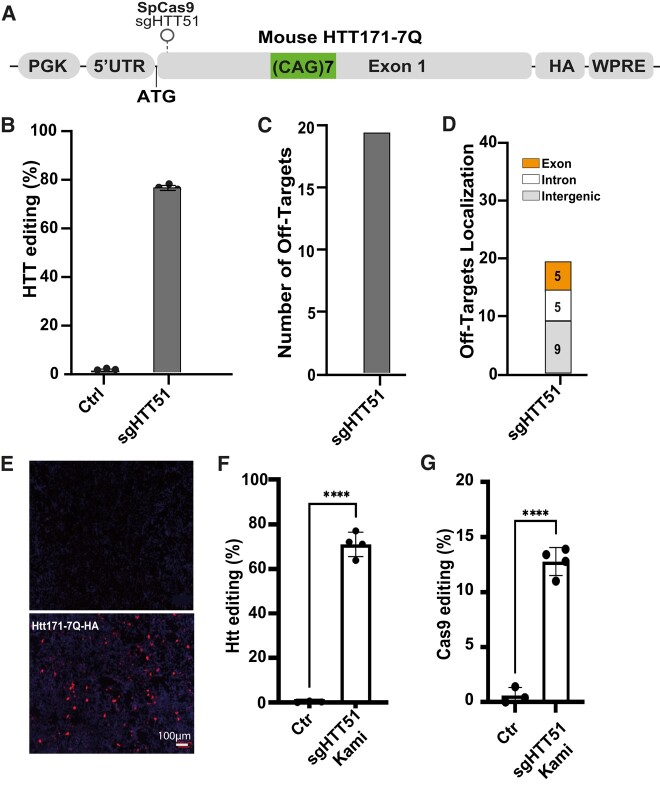
Figure 2.
Design and validation of sgRNA inactivating the mouse Htt gene. (A) Schematic representation of the mouse Htt reporter plasmid used for validation of the various sgRNAs. The plasmid contains the 5′ untranslated region (5′UTR) part of the Htt gene and the codons encoding the first 171 amino acids, including the seven glutamine (codons CAG or CAA) repeats ((CAG)2CAA(CAG)4). The expression of this transgene is under the control of the PGK promoter. An HA-tag was in the 3′ region was used to facilitate the detection of this N-terminal fragment of the Htt protein by western blotting. Finally, the woodchuck post-regulatory element (WPRE) was used to increase transgene expression. The position of the sgRNA targeting the translation start site of the mouse Htt is indicated: sgHTT51. (B) Editing efficiency of sgHTT51 according to TIDE, 3 days after the transfection of HEK293T cells (n = 3 samples/group). (C) A bioinformatic analysis was performed to evaluate the presence of potential off-target (OT) sites for sgHTT51 binding in exons, introns and intergenic regions of the mouse genome. (D) In total, 19 off-target sites were detected for sgHTT51. All those with the most relevant scores were located in intronic or intergenic regions. (E) Immunofluorescence analysis with a mouse monoclonal HA antibody showing the loss of the mouse Htt protein following gene editing in HEK293T cells. (F and G) Final validation of the second-generation KamiCas9 system in mouse primary striatal cultures transduced with the corresponding lentiviral vectors. Very efficient editing of the endogenous mouse Htt gene (F) and Streptococcus pyogenes Cas9 (spCas9) nuclease (G) was observed 12 days post-transduction (n = 4 samples/group). Results are presented as the mean ± SD. Statistics: two-tailed t-test (HTT: t(21.91), df(5)5; Cas9: t(14), df(5)). ****P < 0.0001; ***P < 0.001; **P < 0.01.