Figure 8.
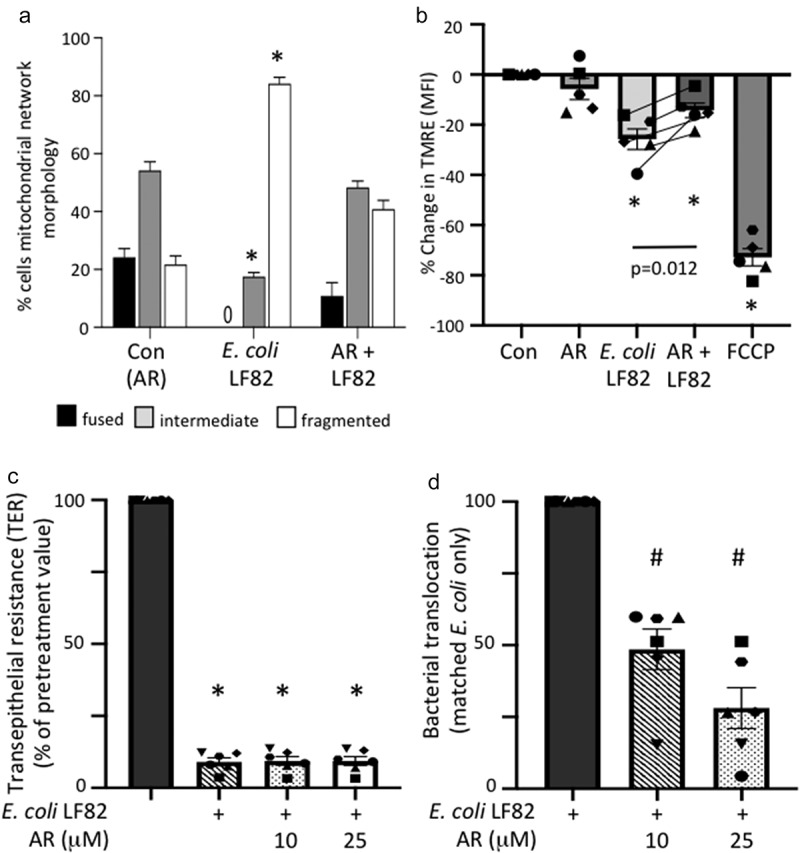
An FFAR3 agonist improves mitochondrial network connectivity and barrier function in E. coli-LF82-infected T84 epithelial cells. Monolayers of the human colon-derived T84 epithelial cell line were treated with E. coli-LF82 (108 cfu, 4 h) ± a co-treatment with the FFAR3 agonist AR420626 (25 µM) and representative images collected in a random fashion by first identifying epithelia nuclei (blue, n) and then swapping the confocal laser channel to assess the mitochondrial network as defined by TOMM-20 immunostains. Twenty cells per monolayer were characterized by semi-quantitative assessment (a). (b) Mitochondrial membrane potential was assessed by TMRE fluorescence in a flow cytometer. A 10 min treatment with the metabolic toxin, FCCP (10 µM, n = 5 epithelial monolayers from separate experiments (indicated by different symbols)). Filter-grown T84 cell monolayers (starting transepithelial resistance (TER) range = 957–2155 Ohms.cm2) were cultured with E. coli-LF82 (108 cfu) ± AR420626 and TER and transcytosis of the bacteria assessed 24 h later. (c) TER is presented as the change over 24 h with each monolayer being its’ own control (i.e., pre-treatment value). (d) Bacterial transcytosis was assessed via serial dilution of culture-well basolateral medium on agar plates, with the data being converted to % transcytosis based on bacterial counts in the apical compartment and then E. coli-LF82 was normalized to 100 for comparison with E. coli+AR420626 in the same experiment (data are mean ± SEM; each data point is an individual experiment (n = 6) in which measurements from 3 or 4 monolayers were averaged and are shown as a different symbol; * and #, p <.05 compared to control uninfected cells (con) and E. coli-LF82 only infected cells, respectively).
