Abstract
Objective
Age is the strongest contributor to 10-year predicted atherosclerotic cardiovascular disease (ASCVD) risk. Some older adults have a predicted ASCVD risk ≥7.5 %, without established risk factors. We sought to compare ASCVD incidence among adults with predicted ASCVD risk ≥7.5 %, with and without established ASCVD risk factors, to adults with predicted risk <7.5 %.
Methods
We analyzed data from REasons for Geographic and Racial Differences in Stroke study participants, 45–79 years old, without ASCVD or diabetes, not taking statins and with low-density lipoprotein cholesterol 70–189 mg/dL. Participants were categorized into 3 groups based on their 10-year predicted ASCVD risk and presence of established risk factors: <7.5 %, ≥7.5 % with established risk factors and ≥7.5 % without established risk factors. Established risk factors included smoking, systolic blood pressure ≥130 mmHg or antihypertensive medication use, total cholesterol ≥200 mg/dL, or high-density lipoprotein cholesterol <50 mg/dL for women (<40 mg/dL for men). Participants were followed for ASCVD events.
Results
Among 11,115 participants, 911 incident ASCVD events occurred over a median of 11.1 years. ASCVD incidence rates were 3.6, 12.8, and 9.8 per 1,000 person-years for participants with predicted risk <7.5 %, predicted risk ≥7.5 % with established risk factors and predicted risk ≥7.5 % without established risk factors, respectively. Compared to adults with predicted risk <7.5 %, hazard ratios for incident ASCVD in participants with risk ≥7.5 % with and without established risk factors were 3.58 (95 %CI 3.03 – 4.21) and 2.72 (95 %CI 1.91–3.88), respectively.
Conclusions
Adults with a 10-year predicted ASCVD risk ≥7.5 % but without established risk factors had a high ASCVD incidence.
Keywords: Cohort study, Preventative cardiology, ASCVD risk
Graphical abstract
1. Introduction
The estimation of atherosclerotic cardiovascular disease (ASCVD) risk with the Pooled Cohort Equations (PCE) is a key step when making decisions for primary prevention [1,2]. Initiation of statin therapy is recommended for adults ages 40 to 75 years with diabetes, low-density lipoprotein cholesterol (LDL-C) ≥190 mg/dL, or LDL-C 70 to 189 mg/dL and a 10-year predicted ASCVD risk ≥7.5 % after considering the presence of selected “risk enhancers” [2]. Age is the strongest contributor to 10-year predicted ASCVD risk [3]. As a result, some older adults have a 10-year predicted ASCVD risk ≥7.5 %, even without established risk factors (i.e.: hypertension, dyslipidemia, and diabetes) [4]. For example, an adult without diabetes mellitus, who does not smoke, with a total cholesterol of 150 mg/dL, high-density lipoprotein cholesterol (HDL-C) of 50 mg/dL, and systolic and diastolic blood pressure of 110/60 mm Hg without the use of antihypertensive medication exceeds a 10-year predicted ASCVD risk ≥7.5 % at 65 years for White men, 67 years for Black men, 71 years for White women and 72 years for Black women. Some have raised concerns that older individuals who meet thresholds for statin treatment primarily because of age when using the PCE may derive limited benefit [5].
Several observational studies have demonstrated a low incidence of ASCVD events among adults without established risk factors [6]. However, individuals with higher vs lower predicted ASCVD risk have a larger absolute risk reduction with the initiation of lipid-lowering medication, regardless of LDL-C levels [3,7,8]. Therefore, it is important to estimate the ASCVD event rates for adults with ASCVD risk ≥ 7.5 % without established risk factors. If adults with ASCVD risk ≥ 7.5 % without established risk factors do not experience high event rates, then they may derive little benefit from initiating a statin. In contrast, if adults with ASCVD risk ≥ 7.5 % without established risk factors experience a high ASCVD event rate, then initiating a statin may be of benefit.
Using data from the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort study, we compared the incidence of ASCVD events among adults with 10-year predicted ASCVD risk ≥ 7.5 % who had established risk factors, adults with ASCVD risk ≥ 7.5 % who did not have established risk factors, and adults with ASCVD risk <7.5 %, regardless of the presence of established risk factors. We further investigated whether some ASCVD risk factors not included in the PCE, referred hereafter as “risk modifiers”, were associated with incident ASCVD events within each of these three risk groups [2].
2. Methods
2.1. Study population
The REGARDS study has been described previously [9]. A total of 30,239 participants aged ≥ 45 years were enrolled from all 48 contiguous United States and the District of Columbia between January 2003 and October 2007. By design, participants living in the “stroke buckle” and “stroke belt” (North Carolina, South Carolina, Georgia, Alabama, Mississippi, Tennessee, Arkansas, and Louisiana) and Black adults were oversampled. All participants completed a computer-assisted telephone interview followed by an in-home examination at baseline. The REGARDS study protocol was approved by the institutional review boards at the participating centers. All participants provided written informed consent.
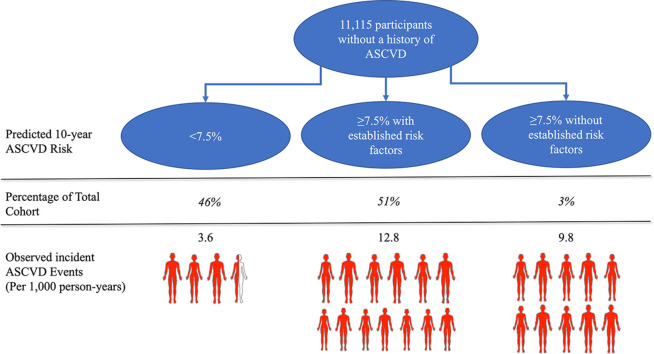
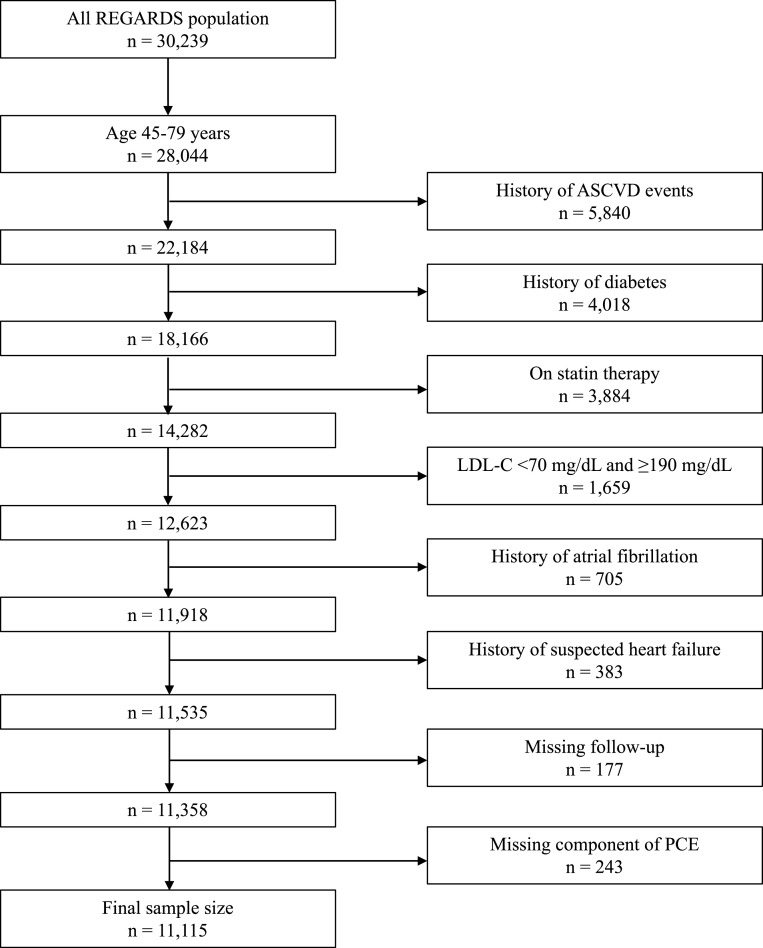
For the present analysis, we included REGARDS study participants ages 45 to 79 years, the upper age range of the ASCVD PCE, without a history of ASCVD or diabetes mellitus who were not taking a statin and had fasting LDL-C between 70 and 189 mg/dL (n = 12,623). Adults with diabetes or LDL-C ≥ 190 mg/dl were excluded as statins are recommended in this group regardless of their 10-year predicted ASCVD risk. Participants with atrial fibrillation (n = 705) or suspected heart failure (n = 383) were excluded because individuals with these conditions were not included in the population used to develop the PCE [1]. Participants without follow-up (n = 177) or missing data on the PCE components (n = 243) were also excluded. After these exclusions, 11,115 participants were included in the current analysis (Fig. 1).
Fig. 1.
Exclusion cascade for analysis of 10-year predicted ASCVD risk with and without established risk factors and association with incident ASCVD events.
ASCVD, atherosclerotic cardiovascular disease; LDL-C, low-density lipoprotein cholesterol; PCE, pooled cohort equations.
2.2. Baseline data collection
Self-reported information on age, sex, race, region of residence, education, annual household income, cigarette smoking, alcohol use, physical activity, history of diabetes, atrial fibrillation, stroke, coronary heart disease [CHD], and antihypertensive medication use was collected by trained staff through a computer-assisted telephone interview. Participants were asked to fast overnight prior to completing an in-home examination. During the examination, weight, height, waist circumference and blood pressure were measured, blood and spot urine samples were collected, and an electrocardiogram (ECG) was performed. Additionally, the names of all prescription and over-the-counter medications taken during the 2 weeks prior to the in-home visit were recorded based on a pill bottle review. Weight and height were used to calculate body mass index (BMI). Abdominal obesity was defined as a waist circumference ≥ 88 cm among women and ≥ 102 cm among men. Total cholesterol, high-density lipoprotein cholesterol (HDL-C), and triglycerides were measured using blood samples by colorimetric reflectance spectrophotometry. LDL-C was calculated using total cholesterol, HDL-C, and triglycerides using the Sampson equation [10]. High-sensitivity C-reactive protein (hs-CRP) and cystatin C were measured by particle-enhanced immunonephelometry. Albuminuria was defined as an urine albumin to urine creatinine ratio ≥ 30 mg/g. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation [11].
A history of ASCVD at baseline was defined as self‐report of a physician diagnosis of myocardial infarction (MI) or stroke; a self‐report of prior coronary artery bypass, coronary angioplasty, or stenting; a lower extremity revascularization procedure or an aortic aneurysm repair surgery; or evidence of a previous MI on the study ECG. Diabetes was defined by a fasting serum glucose level ≥ 126 mg/dL, a random serum glucose of ≥ 200 mg/dL for the 1621 (14.6 %) participants who did not fast for a minimum of 8 h, or self-report of a prior diagnosis by a medical professional with current use of insulin or oral glucose-lowering medications. Prediabetes was defined as fasting serum glucose between 100 and 125 mg/dL or a random serum glucose between 140 and 199 mg/dL among those without diabetes [12]. Atrial fibrillation was defined by self-report or electrocardiogram evidence. Participants were considered to have suspected heart failure if they were taking digoxin in the absence of atrial fibrillation, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker plus beta-blocker in the absence of hypertension, carvedilol, spironolactone, loop diuretics, and/or a combination of hydralazine and nitrates as previously reported [13]. Statin use was ascertained through medication inventory or by self-reported use of lipid-lowering medications. Diet was assessed using a food frequency questionnaire about the usual dietary intake of each participant over the last year. Physical activity was assessed with a questionnaire to determine the number of times per week vigorous physical activity was performed. Diet and physical activity were categorized into ideal, intermediate, and poor levels based on the AHA Life's Simple 7, as reported previously [14].
2.3. Outcome
The outcome of interest was incident ASCVD. Following the in-home visit, participants or their proxies were contacted twice a year via telephone to identify potential incident ASCVD events. Incident ASCVD was defined as the first event of a nonfatal or fatal stroke or CHD, including nonfatal MI or CHD death event. For MI, medical records were examined for the presence of signs or symptoms suggestive of ischemia; a rising and/or falling pattern in cardiac troponin level or creatine phosphokinase-MB level over 6 or more hours with a peak level greater than twice the upper limit of normal; and electrocardiogram changes consistent with ischemia or MI, guided by the Minnesota code [15,16]. For stroke, medical records were retrieved for adjudication when stroke symptoms with a subsequent hospitalization, stroke, or heart-related hospitalization were reported. A committee of experts adjudicated strokes according to the World Health Organization definition as “rapidly developing clinical signs of focal, at times global, disturbance of cerebral function, lasting more than 24 h or leading to death with no apparent cause other than that of vascular origin” [17,18]. When deaths were reported, interviews with proxies, medical records in the last year of life, death certificates, and autopsy reports were used to determine whether stroke or CHD was the main underlying cause. We used follow-up data for CHD and stroke events through December 31, 2017.
2.4. ASCVD risk groups
Each participant's 10-year ASCVD risk was calculated using the sex- and race-specific PCE. Participants were categorized based on their ASCVD risk: <7.5 %, ≥ 7.5 % with established risk factors, and ≥ 7.5 % without established risk factors. Participants in the risk ≥ 7.5 % without established risk factors were non-smokers with systolic blood pressure (SBP) <130 mm Hg and not taking antihypertensive medication, and had total cholesterol <200 mg/dL, and HDL-C ≥ 50 mg/dL for women or ≥ 40 mg/dL for men. An SBP cutoff of 130 mm Hg was used as this is the level at which antihypertensive medication is recommended in the 2017 ACC/AHA guidelines on the management of blood pressure [19].
2.5. Statistical analysis
Baseline characteristics and the incidence rate per 1000 person years along with 95 % confidence intervals (CI) for composite ASCVD events were calculated for participants in each of the three ASCVD risk groups. Crude hazard ratios (HR) and 95 % CI for the association between the risk ≥ 7.5 % with and without established risk factors groups and incident ASCVD events were estimated using Cox proportional hazard models. The ASCVD risk <7.5 % group served as the reference. The proportional hazards assumption was confirmed using the Schoenfeld residual method. The analysis was conducted for the overall population and stratified by sex. A secondary analysis was performed comparing the risk < 7.5 % and risk ≥ 7.5 % without established risk factors groups using participants in the risk ≥ 7.5 % with established risk factors group as the reference. As adults with and without established risk factors groups may have a different distribution of estimated ASCVD risk, we further stratified participants into smaller estimated risk categories (10-year predicted ASCVD risk groups <5 %, 5 % to <7.5 %, 7.5 % to <10 %, 10 % to <15 %, 15 % to <20 %, and ≥ 20 %). Incidence rates and HRs, with 10-year predicted ASCVD risk 5 to <7.5 % serving as the reference, were then calculated within each subgroup. Hazard ratios were not adjusted for traditional risk factors given that these variables were already incorporated into the PCE. Additionally, results were not adjusted for socioeconomic demographics (i.e. income, education, insurance status) due inability to standardize these measures into clinical care. In a sensitivity analysis, we used an SBP <120 mm Hg without the use of antihypertensive medication, which is considered a normal BP, as the BP criterion in defining risk ≥ 7.5 % without established risk factors group and the incidence rates and HRs were recalculated [19].
2.6. Risk modifiers
Levels of ASCVD risk modifiers not included in the PCE but that could influence ASCVD risk were measured, including diet, physical activity, alcohol use, BMI, eGFR, cystatin C, LDL-C, hs-CRP, triglycerides, albuminuria, and pre-diabetes. The levels were estimated for participants within the three 10-year predicted ASCVD risk groups. Elevated LDL-C, hs-CRP, and triglycerides were also assessed in our study as risk modifiers as they are highlighted in the most recent guidelines [2]. Using t-tests or chi-square tests we compared the levels of each risk factor between those who did and did not experience an ASCVD event during follow-up. Two-sided P values of < 0.05 were considered statistically significant. Analyses were conducted using SAS 9.4 (Cary, NC).
3. Results
3.1. Study population and baseline characteristics
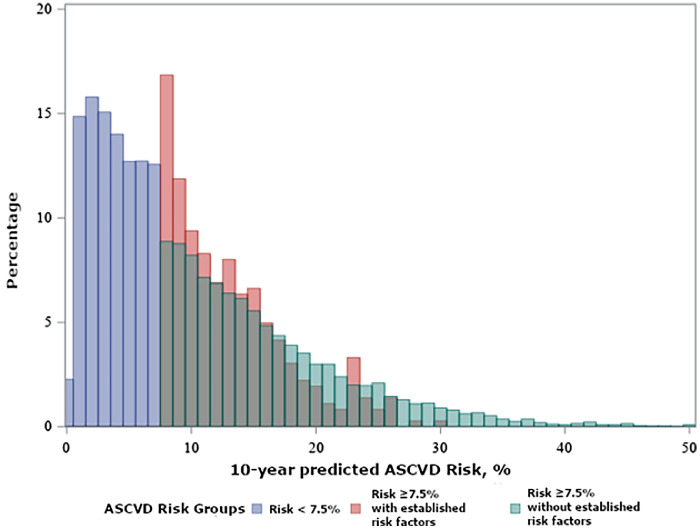
The risk <7.5 %, risk ≥ 7.5 % with established risk factors, and risk ≥ 7.5 % without established risk factors groups included 5116 (46.0 %), 5637 (50.7 %), and 362 (3.3 %) participants with a mean age of 56.3 (standard deviation [SD] 5.9), 66.3 (SD 7.1), and 69.9 (SD 5.1) years respectively (Table 1). Compared to participants with risk ≥ 7.5 % with established risk factors, participants with risk ≥ 7.5 % without established risk factors were more likely to be male, have a BMI < 25 kg/m2, an ideal/intermediate diet, and have an ideal physical activity level. Ideal or intermediate diet scores were observed in fewer than ¼ of the participants, with the smallest proportion in the risk ≥ 7.5 % with established risk factors group (Table 1). The risk ≥ 7.5 % without established risk factors group had the highest proportion of adults with ideal physical activity scores (Table 1). The risk ≥ 7.5 % without established risk factors had a lower percentage of black participants (22.9 %) compared to the risk <7.5 % (32.4 %) and the risk ≥ 7.5 % with established risk factors (41.9 %). Participant characteristics when 10-year predicted risk groups were further divided into smaller subgroups are presented in Supplemental Table 1. Predicted 10-year ASCVD risk overlapped between those in risk ≥ 7.5 % with and without established factors groups (Fig. 2).
Table 1.
Baseline characteristics* of participants by ASCVD risk group.
| Participant characteristics | Risk <7.5 % (n = 5116) | Risk ≥ 7.5 % with established risk factors (n = 5637) | Risk ≥ 7.5 % without established risk factors (n = 362) |
|---|---|---|---|
| Age, years | 56.2 (5.9) | 66.3 (7.1) | 69.9 (5.1) |
| Men, % | 21.9 | 57.2 | 76.2 |
| Black, % | 32.4 | 41.9 | 22.9 |
| Geographic region, % | |||
| Stroke belt | 34.7 | 35.7 | 27.9 |
| Stroke buckle | 23.1 | 17.8 | 17.7 |
| Other US regions | 42.2 | 46.5 | 54.4 |
| Less than high school education, % | 4.5 | 11.5 | 5.3 |
| Annual household income <$25,000, % | 18.4 | 29.4 | 15.7 |
| Body mass index (kg/m2), % | |||
| Normal (<25) | 32.4 | 25.6 | 46.7 |
| Overweight (25 to <30) | 35.2 | 40.7 | 40.0 |
| Obese (≥ 30) | 32.4 | 33.7 | 14.4 |
| Abdominal obesity present, % | 39.3 | 43.1 | 20.7 |
| Current smoker, % | 9.4 | 21.3 | 0.0 |
| Alcohol drinker, % | |||
| None | 55.8 | 52.8 | 59.5 |
| Moderate | 39.3 | 42.0 | 35.8 |
| Heavy | 4.8 | 5.2 | 4.7 |
| Diet Score | |||
| Ideal/Intermediate | 22.9 | 17.9 | 22.8 |
| Poor | 77.1 | 82.1 | 77.2 |
| Physical activity score, % | |||
| Ideal | 30.2 | 33.0 | 41.9 |
| Intermediate | 41.9 | 36.9 | 32.2 |
| Poor | 27.9 | 30.1 | 25.8 |
| Total cholesterol, mg/dL | 202 (31) | 204 (32) | 179 (15) |
| LDL-C, mg/dL | 123.9 (27.0) | 129.4 (26.9) | 107.4 (15.1) |
| Triglycerides, mg/dL | 112.4 (63.2) | 135.4 (79.2) | 92.6 (40.1) |
| HDL-C, mg/dL | 58 (16) | 51 (16) | 55 (11) |
| Cystatin C, mg/L | 0.9 (0.2) | 1.0 (0.3) | 0.9 (0.2) |
| LDL-C ≥ 160 mg/dL, % | 11.2 | 14.6 | 0.0 |
| hs-CRP ≥ 2 mg/L, % | 48.0 | 54.3 | 39.5 |
| Triglycerides ≥ 150 mg/dL, % | 19.1 | 30.1 | 7.5 |
| eGFR < 60 mL/min/1.73m2, % | 1.8 | 7.6 | 3.9 |
| Albuminuria, % | 5.4 | 11.3 | 5.9 |
| Prediabetes, % | 15.8 | 23.0 | 16.6 |
| Systolic blood pressure, mm Hg | 118 (13) | 132 (16) | 118 (8) |
| Diastolic blood pressure, mm Hg | 75 (9) | 79 (10) | 72 (8) |
| Antihypertensive medication use, % | 22.9 | 48.8 | 0.0 |
| 10-year predicted ASCVD risk % | 3.6 [2.0–5.5] | 14.1 [10.4–19.6] | 12.0 [9.3–15.6] |
ASCVD, atherosclerotic cardiovascular disease; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; hs-CRP, high sensitivity C-reactive protein; eGFR, estimated glomerular filtration rate; ASCVD, atherosclerotic cardiovascular disease.
Results reported as mean (standard deviation) or median (inter-quartile range) for 10-year predicted atherosclerotic cardiovascular disease risk as it was not non-normally distributed.
Fig. 2.
Distribution of 10-year predicted atherosclerotic risk by ASCVD risk groups.
ASCVD: Atherosclerotic cardiovascular disease.
3.2. Incident cardiovascular events
A total of 911 incident ASCVD events occurred over a median follow-up of 11.1 years. The incidence rate per 1000 person-years among the risk <7.5 % group was 3.6 (95 % CI 3.1 to 4.1), compared to 12.8 (95 % CI 11.8 to 13.7) in the risk ≥ 7.5 % with established risk factors group and 9.8 (95 % CI 6.9 to 13.2) in the risk ≥ 7.5 % without established risk factors group (Central Illustration). Adults in the risk ≥ 7.5 % with established risk factors group had a HR of 3.58 (95 % CI 3.03 to 4.21), and the risk ≥ 7.5 % without established risk factors group had a HR of 2.72 (95 % CI 1.91 to 3.88), each compared to the risk <7.5 % group (Table 2). When using the risk ≥ 7.5 % with established risk factors as the reference group, the HR for ASCVD events was 0.76 (95 % CI 0.55 to 1.06) for the risk ≥ 7.5 % without established risk factors group. The risk ≥ 7.5 % with established risk factors group had a HR of 3.58 (95 % CI 2.91 to 4.41) while the risk ≥ 7.5 % without established risk factors group had a HR of 2.62 (95 % CI 1.29 to 5.37) compared to the risk <7.5 % group in women. In men, the risk ≥ 7.5 % with established risk factors group had a HR of 3.07 (95 % CI 2.29 to 4.11) and the risk ≥ 7.5 % without established risk factors group had a HR of 2.31 (95 % CI 1.46 to 4.11) compared to the risk <7.5 % group (Supplemental Table 2).
Table 2.
Incidence rates and hazard ratios for atherosclerotic cardiovascular disease associated with 10-year predicted risk groups.
| Risk < 7.5 % | Risk ≥ 7.5 % with established risk factors | Risk ≥ 7.5 % without established risk factors | |
|---|---|---|---|
| Number of participants | 5116 | 5637 | 362 |
| ASCVD events, n ( %) | 183 (3.6) | 691 (12.3) | 37 (10.2) |
| CHD events, n ( %) | 107 (2.1) | 371 (6.6) | 19 (5.3) |
| Stroke event, n ( %) | 79 (1.5) | 324 (5.8) | 18 (4.9) |
| Person-years of follow-up | 51,402.4 | 54,142.3 | 3765.6 |
| Incidence rate per 1000 person-years (95 % CI) | 3.6 (3.1–4.1) | 12.8 (11.8–13.7) | 9.8 (6.9–13.2) |
| Hazard ratio (95 % CI) | 1 (reference) | 3.58 (3.03–4.21) | 2.72 (1.91–3.88) |
| Hazard ratio (95 % CI) | 0.28 (0.24–0.33) | 1 (reference) | 0.76 (0.55–1.06) |
ASCVD, atherosclerotic cardiovascular disease.
There were n = 7 participants who had both stroke and CHD events on same date and have been included for both stroke and CHD event rows. Therefore, the number and percents may be more than the total number and percent of ASCVD events.
The incidence rates in the risk ≥ 7.5 % with established risk factors were 6.3, 10.3, 14.1, and 22.2 per 1000 person-years for participants with a 10-year predicted ASCVD risk of 7.5 % to <10 %, 10 % to <15 %, 15 % to <20 %, and ≥ 20 %, respectively (Supplemental Table 3). The incidence rates in the risk ≥ 7.5 % without established risk factors were 7.6, 8.4, 11.3, and 22.0 per 1000 person-years for participants with a 10-year predicted ASCVD risk of 7.5 % to <10 %, 10 % to <15 %, 15 % to <20 %, and ≥ 20 %, respectively. The incidence rates of the risk <5 % and risk 5 % to <7.5 % was 2.6 and 5.6 respectively. Within each subgroup of estimated ASCVD risk, the HR for incident ASCVD is reported in both the risk ≥ 7.5 % with and without established risk factors groups and presented in Supplemental Table 3.
3.3. Sensitivity analysis
When requiring an SBP <120 mm Hg without the use of antihypertensive medication for the risk ≥ 7.5 % without established risk factors group, 1.6 % of participants met the definition of having risk ≥ 7.5 % without established risk factors. The incidence rates were 3.6 (95 % CI 3.1 to 4.1), 12.7 (95 % CI 11.8 to 13.6), and 9.1 (95 % CI 5.3 to 13.9) per 1000 person-years for the risk <7.5 %, and risk ≥ 7.5 % with and without established risk factors groups, respectively (Supplemental Table 4). The HRs for ASCVD were 3.55 (95 % CI 3.02 to 4.18) and 2.52 (95 % CI 1.53 to 4.14) for the risk ≥ 7.5 % with and without established risk factors, respectively, using the risk <7.5 % group as reference.
3.4. Association of risk modifiers with ASCVD risk
In the risk <7.5 % group, participants who experienced an incident ASCVD event during follow-up were more likely to have an eGFR <60 mL/min/1.73m2 and higher cystatin C level (Table 3). In the risk ≥ 7.5 % with established risk factors group, participants who had ASCVD events during follow-up were more likely to have an eGFR <60 mL/min/1.73m2, higher cystatin C, hs-CRP ≥ 2 mg/L, triglycerides ≥ 150 mg/dL, and albuminuria. There was no statistical difference in risk modifiers between those that did and did not experience ASCVD events in the risk ≥ 7.5 % without established risk factors group.
Table 3.
Risk modifiers among participants who experienced and did not experience an incident atherosclerotic cardiovascular disease event by ASCVD risk group.
| Risk < 7.5 % |
Risk ≥ 7.5 % with established risk factors |
Risk ≥ 7.5 % without established risk factors |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No ASCVD Events (n = 4933) | ASCVD Events (n = 183) | p-value | No ASCVD Events (n = 4946) | ASCVD Events (n = 691) | p-value | No ASCVD Events (n = 325) | ASCVD Events (n = 37) | p-value | |
| Diet score | |||||||||
| Ideal/Intermediate | 23 % | 20 % | 0.400 | 18 % | 17 % | 0.468 | 24 % | 13 % | 0.193 |
| Poor | 77 % | 80 % | 82 % | 83 % | 76 % | 87 % | |||
| Physical Activity Score | |||||||||
| Ideal | 30 % | 31 % | 0.635 | 33 % | 35 % | 0.476 | 43 % | 37 % | 0.353 |
| Intermediate | 42 % | 39 % | 37 % | 35 % | 31 % | 43 % | |||
| Poor | 28 % | 30 % | 30 % | 30 % | 27 % | 20 % | |||
| Alcohol drinker, % | |||||||||
| None | 56 % | 59 % | 0.113 | 52 % | 62 % | 0.486 | 59 % | 62 % | 0.343 |
| Moderate | 40 % | 34 % | 43 % | 32 % | 36 % | 34 % | |||
| Heavy | 5 % | 7 % | 5 % | 6 % | 5 % | 4 % | |||
| Body mass index (kg/m2) | |||||||||
| Normal (<25) | 32 % | 33 % | 0.979 | 26 % | 27 % | 0.513 | 47 % | 43 % | 0.611 |
| Overweight (25–<30) | 35 % | 35 % | 41 % | 42 % | 38 % | 46 % | |||
| Obese (≥ 30) | 32 % | 32 % | 34 % | 32 % | 15 % | 11 % | |||
| eGFR <60 mL/min/1.73m2 | 1.7 % | 6.0 % | <0.001 | 7.1 % | 11.3 % | <0.001 | 3.4 % | 8.1 % | 0.163 |
| Cystatin C, mg/L* | 0.9 (0.2) | 1.0 (0.6) | 0.013 | 1.0 (0.3) | 1.1 (0.3) | <0.001 | 0.9 (0.2) | 0.9 (0.2) | 0.374 |
| LDL-C ≥ 160 mg/dL | 11 % | 13 % | 0.550 | 15 % | 13 % | 0.226 | 0 % | 0 % | – |
| hs-CRP ≥ 2 mg/L | 48 % | 51 % | 0.479 | 54 % | 59 % | 0.005 | 40 % | 38 % | 0.828 |
| Triglycerides ≥ 150 mg/dL | 19 % | 25 % | 0.055 | 29 % | 35 % | 0.003 | 7 % | 11 % | 0.503 |
| Albuminuria | 5 % | 8 % | 0.072 | 10 % | 19 % | <0.001 | 6 % | 10 % | 0.460 |
| Prediabetes | 16 % | 21 % | 0.062 | 23 % | 22 % | 0.563 | 17 % | 16 % | 0.951 |
ASCVD, atherosclerotic cardiovascular disease.
Numbers for cystatin C represent mean (standard deviation).
4. Discussion
In a large, contemporary, cohort of Black and White U.S. adults, only 3.3 % of participants had a predicted 10-year ASCVD risk ≥ 7.5 % based primarily on age. Those with 10-year predicted risk ≥ 7.5 % both with and without established risk factors had a substantially higher risk for incident ASCVD compared to those with a 10-year predicted risk <7.5 % across the spectrum of predicted ASCVD risk. The results were consistent among women and men and when evaluating a lower threshold of an untreated SBP <120 mm Hg.
It is notable that the risk ≥ 7.5 % without established risk factors group comprised only 3 % of the entire cohort. The percentage of risk ≥ 7.5 % based primarily on age is likely to be even smaller than 3 % in the general population as we excluded those with a history of ASCVD or diabetes, and those already taking a statin. Age is the biggest contributor to ASCVD risk [20], [21]. As a result, many critics of risk estimation for primary prevention cite the reliance on age, and the “inevitability” of statin treatment as potential short-comings [5,22]. However, not only do the current results demonstrate that the group of adults who have risk ≥ 7.5 % primarily because of age is quite small, but also that this group has high ASCVD risk. These results support the concept that age is a powerful risk predictor because it integrates the cumulative exposures to even moderate elevations in risk factors [23], [24], [25]. For example, Liu et al. found that among adults treated with antihypertensive medication, a cumulative SBP exposure of >3000 mmHg-years (i.e. 120 mm Hg over 25 years) was associated with higher left ventricular mass than adults who achieved an SBP <120 mm Hg without medication [26]. Studies of LDL-C have also demonstrated that higher cumulative LDL-C levels are associated with a higher incidence of cardiovascular events [27,28].
It is worth noting that among the adults with risk ≥ 7.5 % primarily because of age, more than three-fourths had poor diet scores and more than half did not meet recommendations for ideal levels of physical activity. The high prevalence of a poor diet and inadequate physical activity within the risk ≥ 7.5 % without established risk factors group supports the hypothesis that their elevated risk derives from long-term accumulated exposures to subclinical risk factors. Multiple observational studies have demonstrated associations between consumption of a heart healthy diet and more physical activity with a lower risk for incident ASCVD, even after adjusting for traditional risk factors [29], [30], [31], [32], [33], [34]. The results from the current study highlight the need for public health measures to help adults maintain ideal cardiovascular health.
The decision to initiate statin therapy for primary prevention in adults who have a 10-year estimated ASCVD risk ≥ 7.5 % based primarily on age can be challenging. Many older adults in this cohort may feel that they do not need a statin since they do not have other established cardiovascular risk factors. However, the current study demonstrated that adults with 10-year predicted ASCVD risk ≥ 7.5 % without established risk factors experience event rates above 7.5 %, the clinical trial-supported threshold for benefit of statin therapy [2]. An elevated ASCVD event rate was present even among adults with predicted risk of 7.5 % to <10 %. Large meta-analyses and retrospective studies in primary prevention populations have shown conflicting results with the use of statins in individuals >75 years old with some showing benefit and others showing no effect [35,36]. The Cholesterol Treatment Trialists’ Collaboration showed a significant association between statin therapy and reduction in major vascular events in the age groups of 66–75 years of age [36]. The Pragmatic Evaluation of Events and Benefits of Lipid-lowering in Older Adults (PREVENTABLE) trial will randomize atorvastatin 40 mg versus placebo in US adults ≥ 75 years without established clinical ASCVD with a primary outcome of dementia and persistent disability free survival (NCT04262206) [37]. The Statin Therapy for Reducing Events in the Elderly (STAREE) trial will randomize atorvastatin 40 mg versus placebo in Australian adults ≥ 70 years without clinical ASCVD with a primary outcome of disability free survival and major cardiovascular events (NCT02099123) [38]. The results of these two randomized controlled trials may provide further clarity on this population.
We examined multiple laboratory biomarkers, including LDL-C, triglycerides, and hs-CRP, that were highlighted in recent guidelines as potentially elevating an individual's estimated ASCVD risk [2]. Among the risk ≥ 7.5 % without established risk factors group, we did not find significant differences in the levels of risk biomarkers between those who did and did not experience ASCVD events, however we may have been underpowered. For example, participants in the risk ≥ 7.5 % without established risk factors group who experienced ASCVD events were nearly twice as likely to have albuminuria (10.8 % % vs 5.7 %, p = 0.46) and more than twice as likely to have an eGFR < 60 mL/min/1.73m2 (8.1 % % vs 3.4 %, p = 0.16) compared to participants who did not experience ASCVD events. Among the risk < 7.5 % and the risk ≥ 7.5 % with established risk factors groups, those who experienced ASCVD events were significantly more likely to have a lower eGFR and higher cystatin C, markers of decreased renal function. The use of coronary artery calcium (CAC) scoring could enhance risk assessment in the risk ≥ 7.5 % without established risk factors subgroup. In a combined analysis of 4778 participants from 3 U.S. cohorts, Yano et al. found that 31 % of adults aged 60 years and older had a CAC score of 0, suggesting that it could help identify older adults with higher and lower ASCVD risk [39]. Further study is warranted to determine whether other subclinical markers of disease, such as abdominal adiposity or high-sensitivity troponin, could help guide risk assessment in older adults without established risk factors.
4.1. Limitations
The current study has several strengths, including its large sample size, extensive data collection at baseline and rigorous follow-up and adjudication of ASCVD events. However, the results of our study should be interpreted in the context of its known and potential limitations. Participants’ 10-year predicted risk was assessed at baseline only. Therefore, some participants who were classified as being without established risk factors may have developed risk factors during follow-up. The modest sample size of participants in the risk ≥ 7.5 % without established risk factors group resulted in wide confidence intervals for some estimates, and we were underpowered to detect differences in risk modifiers among those who did and did not experience ASCVD events. CAC score, a potentially useful risk marker as discussed previously, was unfortunately not available in the REGARDS study. In addition, other factors highlighted in the most recent guidelines as risk modifiers (i.e.: ApoB, pre-mature menopause, pre-eclampsia, significant family history of ASCVD) were not available in the REGARDS study and thus could not be assessed. Lp(a) was available for only a very small percentage of participants included in a case-cohort study and thus was not included for analysis. We excluded participants with diabetes because statins are already indicated for this population. However, this removed many individuals at higher risk of incident ASCVD from the study population. It is likely that the exclusion of these individuals resulted in the under-estimation of risk in the risk ≥ 7.5 % with established risk factors group.
5. Conclusion
In a large, contemporary, cohort of Black and white U.S. adults, only 3 % of participants had 10-year predicted ASCVD risk ≥ 7.5 % in the absence of established risk factors. Among the participants with risk ≥ 7.5 % without established risk factors, the incidence rate of ASCVD events was above the trial-supported threshold in which benefit from statin therapy is seen.
Disclosures
Lisandro D. Colantonio, Paul Muntner and Emily B. Levitan receive grant support from Amgen, Inc. The remaining authors have nothing to disclose.
Data availability
To abide by its obligations with NIH/NINDS and the IRB of the University of Alabama at Birmingham, REGARDS facilitates data sharing through formal data use agreements. Any investigator is welcome to request the REGARDS data and documentation through this process. Requests for data access may be sent to the REGARDS study at regardsadmin@uab.edu.
Author agreement
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. This research project is supported by cooperative agreement U01 NS041588 co-funded by the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute on Aging (NIA), National Institutes of Health, Department of Health and Human Service. We wish to report that Lisandro D. Colantonio, Paul Muntner and Emily B. Levitan receive grant support from Amgen, Inc. None of the remaining authors have anything to disclose.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). He is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author. All the co-authors have approved of the final version of this manuscript including the re-submission and reviewer edits.
CRediT authorship contribution statement
Nathan Kong: Conceptualization, Writing – original draft, Writing – review & editing, Visualization. Swati Sakhuja: Methodology, Formal analysis, Writing – review & editing, Visualization. Lisandro D. Colantonio: Methodology, Writing – review & editing. Emily B. Levitan: Writing – review & editing. Donald M. Lloyd-Jones: Writing – review & editing. Mary Cushman: Writing – review & editing, Funding acquisition. Paul Muntner: Methodology, Writing – review & editing. Tamar S. Polonsky: Conceptualization, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Mary Cushman reports financial support was provided by National Institute of Neurological Disorders and Stroke and the National Institute on Aging (NIA), National Institutes of Health, Department of Health and Human Service. Lisandro Colantonio, Paul Muntner, Emily Levitan reports a relationship with Amgen Inc that includes: funding grants.
Acknowledgments
Acknowledgments
We thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions and further information about the study can be found at http://www.regardsstudy.org.
Sources of funding
This research project is supported by cooperative agreement U01 NS041588 co-funded by the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute on Aging (NIA), National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIA. Representatives of the NINDS were involved in the review of the manuscript but were not directly involved in the collection, management, analysis or interpretation of the data. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at: https://www.uab.edu/soph/regardsstudy/
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajpc.2023.100612.
Appendix. Supplementary materials
References
- 1.Goff D.C., DM Lloyd-Jones, Bennett G., Coady S., D'Agostino R.B., Gibbons R., Greenland P., Lackland D.T., Levy D., O'Donnell C.J., et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 2.Grundy S.M., Stone N.J., Bailey A.L., Beam C., Birtcher K.K., Blumenthal R.S., Braun L.T., de Ferranti S., Faiella-Tommasino J., Forman D.E., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139:e1082–e1143. doi: 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lloyd-Jones D.M., Huffman M.D., Karmali K.N., Sanghavi D.M., Wright J.S., Pelser C., Gulati M., Masoudi F.A., Goff D.C., Jr Estimating longitudinal risks and benefits from cardiovascular preventive therapies among medicare patients: the million hearts longitudinal ASCVD risk assessment tool: a special report from the american heart association and American College of Cardiology. Circulation. 2017;135:e793–e813. doi: 10.1161/cir.0000000000000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karmali K.N., Goff D.C., Jr., Ning H., Lloyd-Jones D.M. A systematic examination of the 2013 ACC/AHA pooled cohort risk assessment tool for atherosclerotic cardiovascular disease. J Am Coll Cardiol. 2014;64:959–968. doi: 10.1016/j.jacc.2014.06.1186. [DOI] [PubMed] [Google Scholar]
- 5.Ridker P.M., Cook N.R. The pooled cohort equations 3 years on. Circulation. 2016;134:1789–1791. doi: 10.1161/CIRCULATIONAHA.116.024246. [DOI] [PubMed] [Google Scholar]
- 6.Daviglus M.L., Stamler J., Pirzada A., Yan L.L., Garside D.B., Liu K., Wang R., Dyer A.R., Lloyd-Jones D.M., Greenland P. Favorable cardiovascular risk profile in young women and long-term risk of cardiovascular and all-cause mortality. JAMA. 2004;292:1588–1592. doi: 10.1001/jama.292.13.1588. [DOI] [PubMed] [Google Scholar]
- 7.Sheppard J.P., Stevens S., Stevens R., Martin U., Mant J., Hobbs F.D.R., McManus R.J. Benefits and harms of antihypertensive treatment in low-risk patients with mild hypertension. JAMA Intern Med. 2018;178:1626–1634. doi: 10.1001/jamainternmed.2018.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohula E.A., Morrow D.A., Giugliano R.P., Blazing M.A., He P., Park J.G., Murphy S.A., White J.A., Kesaniemi Y.A., Pedersen T.R., et al. Atherothrombotic risk stratification and ezetimibe for secondary prevention. J Am Coll Cardiol. 2017;69:911–921. doi: 10.1016/j.jacc.2016.11.070. [DOI] [PubMed] [Google Scholar]
- 9.Howard V.J., Cushman M., Pulley L., Gomez C.R., Go R.C., Prineas R.J., Graham A., Moy C.S., Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 10.Sampson M., Ling C., Sun Q., Harb R., Ashmaig M., Warnick R., Sethi A., Fleming J.K., Otvos J.D., Meeusen J.W., et al. A new equation for calculation of low-density lipoprotein cholesterol in patients with normolipidemia and/or hypertriglyceridemia. JAMA Cardiol. 2020;5:540–548. doi: 10.1001/jamacardio.2020.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey A.S., Stevens L.A. Estimating GFR using the CKD epidemiology collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55:622–627. doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carson A.P., Muntner P., Kissela B.M., Kleindorfer D.O., Howard V.J., Meschia J.F., Williams L.S., Prineas R.J., Howard G., Safford M.M. Association of prediabetes and diabetes with stroke symptoms: the reasons for geographic and racial differences in stroke (REGARDS) study. Diabetes Care. 2012;35:1845–1852. doi: 10.2337/dc11-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goyal P., Mefford M.T., Chen L., Sterling M.R., Durant R.W., Safford M.M., Levitan E.B. Assembling and validating a heart failure-free cohort from the reasons for geographic and racial differences in stroke (REGARDS) study. BMC Med Res Methodol. 2020;20:53. doi: 10.1186/s12874-019-0890-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akinyelure O.P., Sakhuja S., Colvin C.L., Clark D., Jaeger B.C., Hardy S.T., Howard G., Cohen L.P., Irvin M.R., Tanner R., et al. Cardiovascular health and transition from controlled blood pressure to apparent treatment resistant hypertension. Hypertension. 2020;76:1953–1961. doi: 10.1161/HYPERTENSIONAHA.120.15890. [DOI] [PubMed] [Google Scholar]
- 15.Prineas R.J., Crow R.S., Zhang Z.M. 2nd ed. Springer; London: 2010. The Minnesota code manual of electrocardiographic findings: including measurement and comparison with the Novacode; standards and procedures for ECG measurement in epidemiologic and clinical trials. [Google Scholar]
- 16.Safford M.M., Brown T.M., Muntner P.M., Durant R.W., Glasser S., Halanych J.H., Shikany J.M., Prineas R.J., Samdarshi T., Bittner V.A., et al. Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308:1768–1774. doi: 10.1001/jama.2012.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stroke–1989. Recommendations on stroke prevention, diagnosis, and therapy Report of the WHO task force on stroke and other cerebrovascular disorders. Stroke. 1989;20:1407–1431. doi: 10.1161/01.str.20.10.1407. [DOI] [PubMed] [Google Scholar]
- 18.Howard V.J., Kleindorfer D.O., Judd S.E., McClure L.A., Safford M.M., Rhodes J.D., Cushman M., Moy C.S., Soliman E.Z., Kissela B.M., et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol. 2011;69:619–627. doi: 10.1002/ana.22385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whelton P.K., Carey R.M., Aronow W.S., Casey D.E., Collins K.J., Himmelfarb C.D., DePalma S.M., Gidding S., Jamerson K.A., Jones D.W., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 20.Bress A.P., Colantonio L.D., Booth J.N., 3rd, Spruill T.M., Ravenell J., Butler M., Shallcross A.J., Seals S.R., Reynolds K., Ogedegbe G., et al. Modifiable risk factors versus age on developing high predicted cardiovascular disease risk in blacks. J Am Heart Assoc. 2017;6 doi: 10.1161/jaha.116.005054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leening M.J.G., Cook N.R., Ridker P.M. Should we reconsider the role of age in treatment allocation for primary prevention of cardiovascular disease? Eur Heart J. 2017;38:1542–1547. doi: 10.1093/eurheartj/ehw287. [DOI] [PubMed] [Google Scholar]
- 22.Kannel W.B., Vasan R.S. Is age really a non-modifiable cardiovascular risk factor? Am J Cardiol. 2009;104:1307–1310. doi: 10.1016/j.amjcard.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sniderman A.D., Furberg C.D. Age as a modifiable risk factor for cardiovascular disease. Lancet. 2008;371:1547–1549. doi: 10.1016/S0140-6736(08)60313-X. [DOI] [PubMed] [Google Scholar]
- 24.Jackson R., Kerr A., Wells S. ‘Should we reconsider the role of age in treatment allocation for primary prevention of cardiovascular disease?’ No, but we can improve risk communication metrics. Eur Heart J. 2017;38:1548–1552. doi: 10.1093/eurheartj/ehw322. [DOI] [PubMed] [Google Scholar]
- 25.Liu K., Colangelo L.A., Daviglus M.L., Goff D.C., Pletcher M., Schreiner P.J., Sibley C.T., Burke G.L., Post W.S., Michos E.D., et al. Can antihypertensive treatment restore the risk of cardiovascular disease to ideal levels? J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Domanski M.J., Tian X., Wu C.O., Reis J.P., Dey A.K., Gu Y., Zhao L., Bae S., Liu K., Hasan A.A., et al. Time course of LDL cholesterol exposure and cardiovascular disease event risk. J Am Coll Cardiol. 2020;76:1507–1516. doi: 10.1016/j.jacc.2020.07.059. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y., Pletcher M.J., Vittinghoff E., Clemons A.M., Jacobs D.R., Jr., Allen N.B., Alonso A., Bellows B.K., Oelsner E.C., Zeki Al Hazzouri A., et al. Association between cumulative low-density lipoprotein cholesterol exposure during young adulthood and middle age and risk of cardiovascular events. JAMA Cardiol. 2021;6:1406–1413. doi: 10.1001/jamacardio.2021.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chomistek A.K., Manson J.E., Stefanick M.L., Lu B., Sands-Lincoln M., Going S.B., Garcia L., Allison M.A., Sims S.T., LaMonte M.J., et al. Relationship of sedentary behavior and physical activity to incident cardiovascular disease: results from the women's health initiative. J Am Coll Cardiol. 2013;61:2346–2354. doi: 10.1016/j.jacc.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Estruch R., Ros E., Salas-Salvadó J., Covas M.I., Corella D., Arós F., Gómez-Gracia E., Ruiz-Gutiérrez V., Fiol M., Lapetra J., et al. Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378:e34. doi: 10.1056/NEJMoa1800389. [DOI] [PubMed] [Google Scholar]
- 30.Reedy J., Krebs-Smith S.M., Miller P.E., Liese A.D., Kahle L.L., Park Y., Subar A.F. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J Nutr. 2014;144:881–889. doi: 10.3945/jn.113.189407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sattelmair J., Pertman J., Ding E.L., Kohl H.W., Haskell W., Lee I.M. Dose response between physical activity and risk of coronary heart disease. Circulation. 2011;124:789–795. doi: 10.1161/CIRCULATIONAHA.110.010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sotos-Prieto M., Bhupathiraju S.N., Mattei J., Fung T.T., Li Y., Pan A., Willett W.C., Rimm E.B., Hu F.B. Association of changes in diet quality with total and cause-specific mortality. N Engl J Med. 2017;377:143–153. doi: 10.1056/NEJMoa1613502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kong N.W., Ning H., Zhong V.W., Paluch A., Wilkins J.T., Lloyd-Jones D., Allen N.B. Association between diet quality and incident cardiovascular disease stratified by body mass index. Am J Prevent Cardiol. 2021;8 doi: 10.1016/j.ajpc.2021.100298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orkaby A.R., Driver J.A., Ho Y.L., Lu B., Costa L., Honerlaw J., Galloway A., Vassy J.L., Forman D.E., Gaziano J.M., et al. Association of statin use with all-cause and cardiovascular mortality in US veterans 75 years and older. JAMA. 2020;324:68–78. doi: 10.1001/jama.2020.7848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407–415. doi: 10.1016/s0140-6736(18)31942-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Golomb B.A., Evans M.A. Statin adverse effects: a review of the literature and evidence for a mitochondrial mechanism. Am J Cardiovasc Drugs: Drugs Devices Other Intervent. 2008;8:373–418. doi: 10.2165/0129784-200808060-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fried T.R., O'Leary J., Towle V., Goldstein M.K., Trentalange M., Martin D.K. Health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. J Am Geriatr Soc. 2014;62:2261–2272. doi: 10.1111/jgs.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoel R.W., Giddings Connolly R.M., Takahashi P.Y. Polypharmacy management in older patients. Mayo Clin Proc. 2021;96:242–256. doi: 10.1016/j.mayocp.2020.06.012. [DOI] [PubMed] [Google Scholar]
- 39.Yano Y., O'Donnell C.J., Kuller L., Kavousi M., Erbel R., Ning H., D'Agostino R., Newman A.B., Nasir K., Hofman A., et al. Association of coronary artery calcium score vs age with cardiovascular risk in older adults: an analysis of pooled population-based studies. JAMA Cardiol. 2017;2:986–994. doi: 10.1001/jamacardio.2017.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
To abide by its obligations with NIH/NINDS and the IRB of the University of Alabama at Birmingham, REGARDS facilitates data sharing through formal data use agreements. Any investigator is welcome to request the REGARDS data and documentation through this process. Requests for data access may be sent to the REGARDS study at regardsadmin@uab.edu.