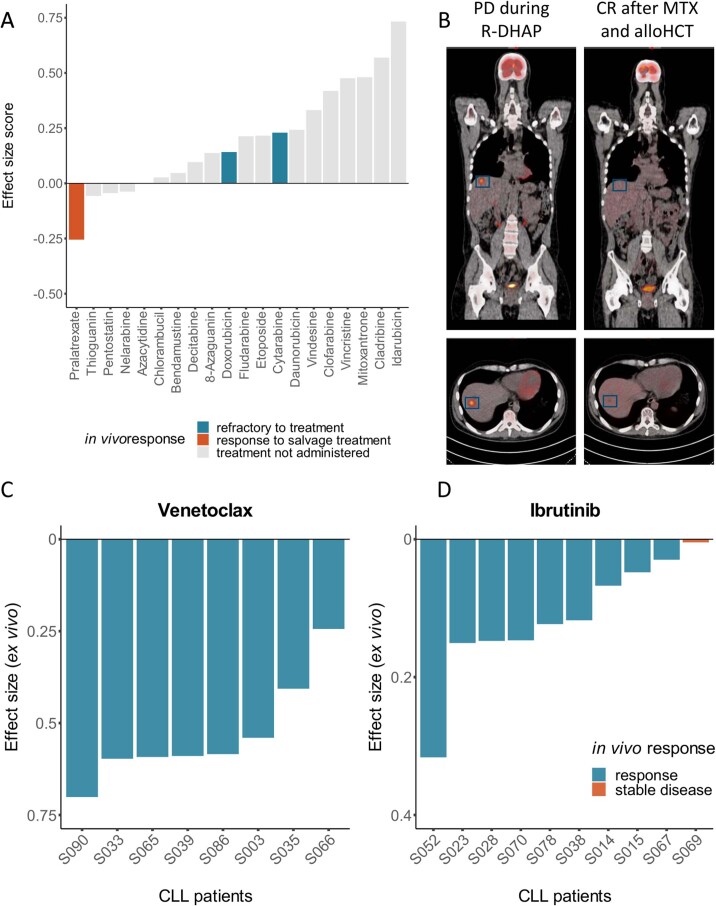
Extended Data Fig. 7. Clinical translation of ex vivo drug response profiling in a patient with highly aggressive refractory Burkitt lymphoma.
(a) Normalized ex vivo effect size scores (ESS) of all chemotherapeutics for S005. The effect size (1- viability AUC) of each drug was subtracted from the median effect sizes of the same drug of the complete SMARTrial study cohort. A negative and positive ESS indicate a higher or lower effect size in this specific patient sample, respectively. S005 was less responsive to the majority of chemotherapeutics than the cohort median ex vivo. The ex vivo effect of pralatrexate was higher than the median effect across all SMARTrial samples. (b) PET-CT scans of participant S005. Left: PET-CT scan showing a progressive disease (PD) in the liver after previous treatment with R-DHAP (rituximab, high dose cytarabine, cisplatin, dexamethasone). Right: PET-CT scan after treatment with 3 cycles of methotrexate (MTX) and consolidating allogeneic hematopoietic cell transplantation (alloHCT). The patient achieved a partial response after treatment with MTX which enabled him to undergo alloHCT and eventually resulted in complete remission. (C + D) Association of in vivo and ex vivo response in CLL patients treated with venetoclax +/- anti- CD20 treatment (c) and ibrutinib (d). The ex vivo drug effect size is defined as 1-viability(AUC). Each bar represents one patient. The color of the bar represents in vivo response.