Fig. 4. Substitution of K+ ion by Li+ increases the activation barrier for the branching reaction.
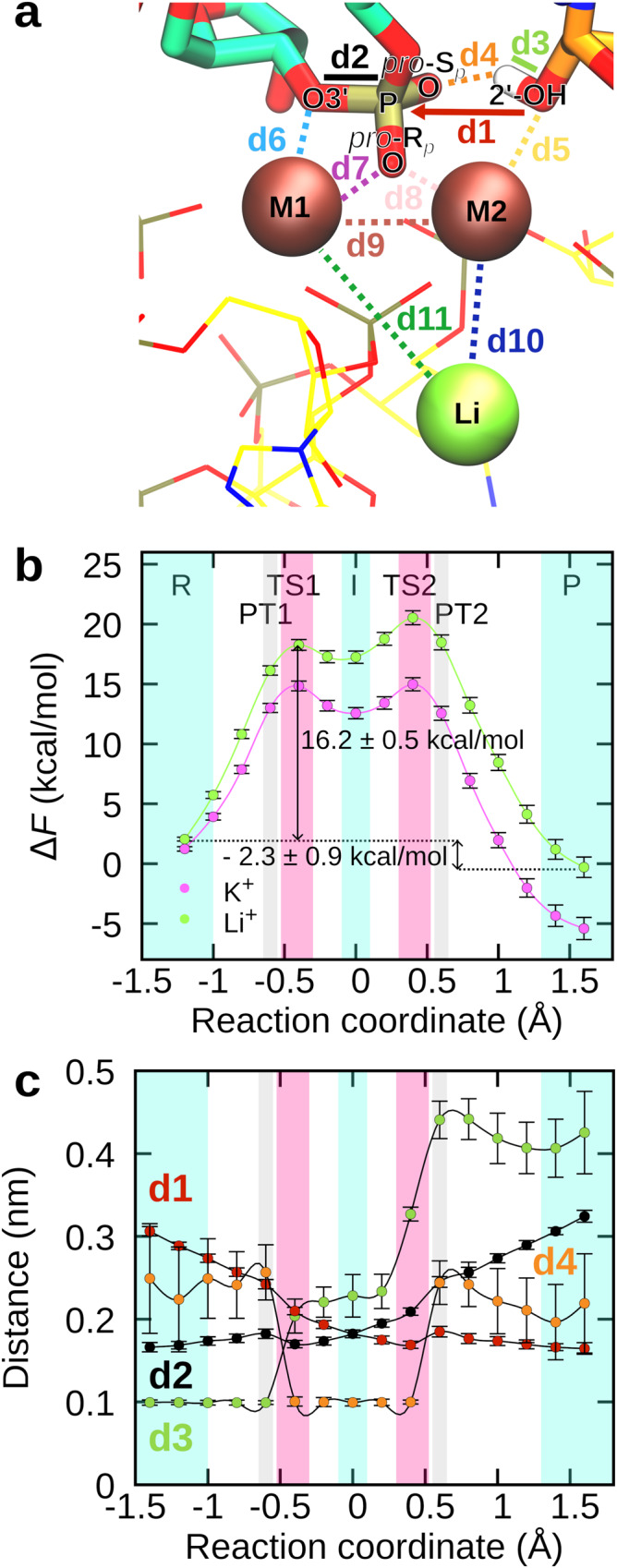
a Active site in the pre-reaction state after equilibration with QM/MM MD. b Helmholtz free energy (F) as a function of the reaction coordinate (RC) in the presence of Li+ ion (in green) in comparison to the free energy profile obtained when K+ ion was bound in the active site instead (in violet). As in the case of the K+ ion, the reaction exhibits an intermediate state (I), two transition states (TS1 and TS2), and two proton transfer steps (PT1 and PT2). The arrows denote the activation barrier and the free energy difference between the reactant (R) and product state (P). The free energy profiles were obtained by integrating the mean constraint force over the RC. At each RC value, the mean constraint force was calculated from the last 6000 frames. The corresponding errors were obtained from SD using error propagation. c Distances between atom pairs depicted in panel a as a function of the reaction coordinate. Data are presented as mean values ± SD (n = 1000 frames). For clarity, only distances exhibiting a marked change during the reaction are shown. Source data are provided as a Source Data file.
