Abstract
In May 2016, the Global Polio Eradication Initiative (GPEI) coordinated the cessation of all use of type 2 oral poliovirus vaccine (OPV2), except for emergency outbreak response. Since then, paralytic polio cases caused by type 2 vaccine-derived polioviruses now exceed 3,000 cases reported by 39 countries. In 2022 (as of April 25, 2023), 20 countries reported detection of cases and 9 other countries reported environmental surveillance detection, but no reported cases. Recent development of a genetically modified type 2 OPV (nOPV2) may help curb the generation of neurovirulent vaccine-derived strains; its use since 2021 under Emergency Use Listing is limited to outbreak response activities. Prior modeling studies showed that the expected trajectory for global type 2 viruses does not appear headed toward eradication, even with the best possible properties of nOPV2 under the current outbreak response performance. Continued persistence of type 2 poliovirus transmission exposes the world to the risks of potentially high-consequence events such as the importation of virus into high transmission areas of India or Bangladesh. Building on prior polio endgame modeling and assuming current national and GPEI outbreak response performance, we show no probability of successfully eradicating type 2 polioviruses in the near term regardless of vaccine choice. We also demonstrate the possible worst-case scenarios could result in rapid expansion of paralytic cases and preclude the goal of permanently ending all cases of poliomyelitis in the foreseeable future. Avoiding such catastrophic scenarios will depend on the development of strategies that raise population immunity to type 2 polioviruses.
Keywords: polio, eradication, dynamic modeling, oral poliovirus vaccine, interdependent risks
1. Introduction
Although the use of live, attenuated oral poliovirus vaccine (OPV) enabled nearly all countries to stop the transmission of wild polioviruses (WPVs), OPV use comes with risks of vaccine-associated paralytic polio (VAPP) and vaccine-derived polioviruses (VDPVs) (Duintjer Tebbens et al., 2006). Consequently, since the early 2000s, coordinated cessation of all use of OPV after successful WPV eradication has been a key component of strategic planning for the polio endgame (Global Polio Eradication Initiative, 2013, 2019, 2020, 2021; World Health Assembly, 2008; World Health Organization, 2010).
In May 2016, the Global Polio Eradication Initiative (GPEI) coordinated the cessation of all use of type 2 OPV (OPV2), except for emergency outbreak response (Hampton et al., 2016). Prior to OPV2 cessation, the GPEI developed extensive OPV2 cessation risk management plans, which included standard operating procedures (SOPs) for outbreak response and the creation of a stockpile of type 2 monovalent OPV (mOPV2). OPV2 cessation planning assumed an understanding of the risks associated with waning population immunity to type 2 polioviruses and the increasing risk of growth and expansion of vaccine-derived type 2 strains, which motivated the development of standard operating procedures for outbreak response (Global Polio Eradication Initiative, 2016). Prior modeling recognized the possibility of needing to restart OPV2 in routine immunization (RI) for adequate control in the event that outbreak response efforts did not succeed (Duintjer Tebbens et al., 2015; Duintjer Tebbens, Pallansch, et al., 2016).
Despite the planning efforts, since May 2016 over 3,000 paralytic polio cases caused by type 2 circulating VDPVs (cVDPV2s) have been reported by 39 countries in different parts of the world (as of April 25, 2023) (Global Polio Eradication Initiative, 2023a; Thompson, 2022b). In 2022 alone, 20 countries reported a total of 673 cases and 9 additional countries reported environmental surveillance detections without cases (as of April 25, 2023) (Global Polio Eradication Initiative, 2023a). To curb the emergence of neurovirulent vaccine-derived strains, the GPEI partners supported the accelerated development of novel OPV2 (nOPV2), which is designed to be more genetically stable than Sabin OPV2 (mOPV2). Since 2021, many countries used nOPV2 under World Health Organization (WHO) Emergency Use Listing (EUL) (Macklin et al., 2023) to respond to cVDPV2 outbreaks. With more than 500 million nOPV2 doses deployed to date (Rachlin et al., 2022), the data on the performance of nOPV2 in the field (e.g., effectiveness, potential to revert) remains preliminary (Martin et al., 2022) and the vaccine is yet to receive a full license. A summary of studies published by June of 2022 (Global Polio Eradication Initiative, 2022), anticipated that nOPV2 clinical results will likely meet expectations as a bioequivalent vaccine compared to Sabin OPV2. However, experience with nOPV2 in the field demonstrates its ability to pose risks of VAPP (World Health Organization, 2023) and cVDPVs (Global Polio Eradication Initiative, 2023b), albeit at lower rates than expected with mOPV2. These lower risks are consistent with nOPV2 increased genetic stability and lower observed shedding, which reduce its risk to individuals and the chances of seeding new outbreaks. These benefits, however, come at the cost of reduced secondary spread and population effectiveness (Thompson, 2022a).
Modeling studies performed before the COVID-19 pandemic showed that GPEI efforts to end cVDPV2 transmission were off track, and found that even assuming the best possible properties of nOPV2, with current GPEI and country outbreak response performance using nOPV2 instead of mOPV2 would not stop cVDPV2 transmission (Kalkowska, Pallansch, Wassilak, et al., 2021; Kalkowska, Pallansch, et al., 2023; Kalkowska, Pallansch, Wilkinson, et al., 2021). Further modeling since COVID-19 explored the consequences of disruptions in RI and polio program activities, and demonstrated the consequences of delaying outbreak response to wait for nOPV2 instead of using mOPV2 (Kalkowska, Pallansch, et al., 2023; Kalkowska, Voorman, et al., 2023; Kalkowska, Wassilak, Pallansch, et al., 2023). Collectively, these modeling studies motivated exploration of conditions that might lead to uncontrollable cVDPV2 outbreaks and further exploration of the potential benefits of nOPV2 considering the bounds of prior analyses (Kalkowska, Pallansch, et al., 2023; Kalkowska, Voorman, et al., 2023; Kalkowska, Wassilak, Pallansch, et al., 2023). Discussion of the expected trajectories from a recent study (Kalkowska, Wassilak, Wiesen, et al., 2023) also led to questions about variability around the expected values and drivers of the upper bounds.
Integrated modeling provides the opportunity to explore prospective outcomes expected with the application of different strategies or policies with full consideration of stochastic risks that reintroduce live polioviruses into populations from different sources (Thompson & Kalkowska, 2020). For example, reintroductions may follow breaches in containment (Duintjer Tebbens, Kalkowska, et al., 2018), unintentional or intentional reintroductions (Kalkowska, Pallansch, Cochi, et al., 2021; Kalkowska, Pallansch, Wassilak, et al., 2021), introductions due to the excretion of polioviruses from individuals transmitting outbreak viruses or type 2 OPV (OPV2) used for outbreak response, or from immunodeficient individuals with prolonged or chronic infections (iVDPVs) (Kalkowska et al., 2019). These events that occur unpredictably in real life are introduced stochastically in the prospective model to vary the times and places where they occur (Kalkowska, Pallansch, Wassilak, et al., 2021). This leads to different possible futures, although the modeling uses the same set of all other inputs to ensure consistent comparisons across policy or strategy scenarios (Kalkowska, Pallansch, Wassilak, et al., 2021).
Policy analyses generally focus on the expected values of outcomes of different strategies to facilitate overall comparisons (Duintjer Tebbens et al., 2015), in which stochastic results vary for different iterations. Although useful for tracking trends and comparing policies, the expected values do not convey the skewness in the distributions caused by high-consequence events, and may miss important associated insights relevant to risk management. For example, prior modeling that explored the specific iterations that led to OPV restart, which the model triggered upon reaching specific cumulative modeled cases, helped to identify specific failure modes (Duintjer Tebbens & Thompson, 2018). Although worst-case scenarios represent low-probability events in the entire simulation space, they can reveal insights about potential catastrophic consequences and provide opportunities for prospective risk management. Building on recent stochastic polio endgame modeling (Kalkowska, Wassilak, Pallansch, et al., 2023; Thompson et al., 2022), we explore what happens in the worst-case iterations of simulations of the polio endgame using different vaccine choices for outbreak response to identify potential high-consequence events that may lead to large numbers of polio cases
2. Methods
For this analysis we use an updated global poliovirus transmission model (Kalkowska, Pallansch, Wassilak, et al., 2021; Kalkowska, Pallansch, Wilkinson, et al., 2021; Kalkowska, Wassilak, Pallansch, et al., 2023) which divides the world according to World Bank Income Level (low-income, LI; lower middle-income, LMI; upper middle-income, UMI; high-income, HI) with current vaccine use in RI (OPV+IPV, IPV/OPV, IPV-only) into 72 blocks of 10 subpopulations of approximately 10.7 million total population and variable age distribution each. The model uses OPV+IPV to refer to the RI schedules of countries that previously relied exclusively on OPV and added one dose of IPV (typically administered at the same time as the third OPV dose) around 2016. This contrasts with sequential IPV/OPV RI schedules, which administer IPV doses at the first individual immunization contacts and then administer OPV at later contacts, that reduce VAPP by giving IPV first. Mixing within blocks occurs homogenously in space and heterogeneously by age. Mixing between blocks occurs according to nine varying preferential mixing areas of different size, which in abstract represent larger geographical regions (e.g., continents) (Kalkowska, Pallansch, Wassilak, et al., 2021; Kalkowska, Pallansch, Wilkinson, et al., 2021; Kalkowska, Wassilak, Pallansch, et al., 2023).
Building on recent analyses that explored the consequences of bOPV cessation in 2027 (Kalkowska, Wassilak, Wiesen, et al., 2023; Thompson et al., 2022), which optimistically assumed eradication of type 1 WPV (WPV1) in 2023 and realistically assumed current SIA performance characteristics (Kalkowska, Badizadegan, et al., 2023; Kalkowska, Pallansch, Wassilak, et al., 2021; Kalkowska, Pallansch, Wilkinson, et al., 2021; Kalkowska, Wassilak, Pallansch, et al., 2023), we used the same realistic SIA performance characteristics for this analysis. The actual performance of SIAs substantially impacts the expected trajectories, as demonstrated elsewhere (Thompson & Kalkowska, 2021; Thompson et al., 2023), which led to emphasis on SIA quality in numerous modeling studies published since 2006 (Thompson et al., 2006) and reviewed elsewhere (Thompson & Kalkowska, 2020). We selected a prospective analytical time horizon of T0 of January 1, 2022 to Tend of December 31, 2035. Recognizing the censoring associated with time horizons, we present the modeling results for both the time horizon of the current GPEI strategic plan of January 1, 2022 to Tend of December 31, 2026, and the full modeled time horizon of January 1, 2022 to Tend of December 31, 2035. For this analysis, we consider three scenarios based on prior modeling (Kalkowska, Wassilak, Pallansch, et al., 2023) that vary the vaccine of choice use for type 2 outbreak response.
Specifically, we consider the scenarios of (i) mOPV2, with its well-established properties from extensive use (ii) best nOPV, which assumes type-specific nOPV use for outbreak response after type-specific OPV cessation, the same effectiveness of nOPV as mOPV, no reversion of nOPV despite transmissibility, and no VAPP, and (iii) worst nOPV, which assumes type-specific nOPV use for outbreak response after type-specific OPV cessation, 90% effectiveness of nOPV relative to mOPV, and reduced reversion based on prior modeling (Kalkowska, Pallansch, Wilkinson, et al., 2021) and for which we further reduced VAPP and reversion rates by 10% relative to mOPV2. Recognizing uncertainty in actual performance of nOPV2, we provide bounding analyses (Kalkowska, Pallansch, Wilkinson, et al., 2021) that convey the probable range. We consider the best nOPV2, which likely performs better than implied by evidence to date for the actual performance of nOPV2 (e.g., due to some VAPP observed (World Health Organization, 2022) and potentially slightly lower efficacy of nOPV2 relative to mOPV2), and the worst nOPV2, which likely performs worse than implied by the evidence from actual nOPV2 use to date. For the extended time horizon, we optimistically assume the potential availability of novel OPV types 1 and 3 at the time of bOPV cessation onward for outbreak response, and we make parallel bounding assumptions for these potential future vaccine products to the ones used for best nOPV2 and worst nOPV2 (Kalkowska, Wassilak, Wiesen, et al., 2023; Thompson et al., 2022).
We performed all simulations using JAVA™ programming language in the integrated development environment Eclipse™, and we simulated 100 stochastic iterations starting with the same random number seeds and initial conditions for each scenario. We estimate the probability of die out (POD) for each scenario by counting the number of iterations with no ongoing transmission of type 2 at the end of the time horizon (Kalkowska, Wassilak, Pallansch, et al., 2023). We demonstrate the general variability among 100 stochastic iterations, and we show the 10 worst performing iterations in terms of cumulative cases of cVDPV2 for each of the model time horizons. We run the model without any restrictions on vaccine supplies to estimate the number of vaccine doses the model would requires under this assumption. We summarize both the expected cases for each iteration and the extent of transmission spread through the 720 modeled subpopulations, and characterize the possible worst-case scenarios of uncontrolled type 2 transmission for each of the modelled scenarios and time horizons.
Although this analysis focuses on type 2 cases, because cVDPV2 cases currently dominate the global case polio counts, using the extended time horizon the analysis also includes assumptions related to the potential risks associated with bOPV cessation (assuming no bOPV intensification prior to cessation and for which type 1 cVDPV risks after cessation become dominant (Kalkowska, Wassilak, Wiesen, et al., 2023), results not shown). We explore the specific subpopulations that contributed to the incidence that led each of the iterations into the 10 highest case counts. We also explored the characteristics of the 10 iterations with the lowest case counts.
3. Results
For the time horizon of 2022–2026, the top row of Table 1 (labeled “All blocks”) shows the model estimates between 6,438 and 22,240 expected cVDPV2 cases in the rightmost columns, depending on the vaccine choice used for outbreak response. Notably, none of the 100 iterations show die out of transmission at the end of the time horizon, which implies an estimated POD of 0% for cVDPV2 (not shown in Table 1 due to 0 value for all vaccine choice scenarios).
Table 1.
High R0 and/or low RI coverage blocks, characteristics, reported and modeled expected cVDPV2 since OPV2 cessation (as of April 25, 2023) and modeled expected cVDPV2 cases for the 2022–2026 time horizon
| Block | IL | Name | R0 | Lowest RI coverage in the block | Reported cVDPV2 cases between May 1, 2016-December 31, 2022* | Modeled expected cVDPV2 cases since OPV2 cessation+ | Modeled expected cVDPV2 cases 2022–2026 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| mOPV2 | best nOPV2 | worst nOPV2 | mOPV2 | best nOPV2 | worst nOPV2 | ||||||
| All Blocks (global) | |||||||||||
| 1–72 | 2973 | 3374 | 3279 | 3393 | 17,138 | 6,438 | 22,240 | ||||
| High R0 (≥10) and low RI coverage (≤0.3) blocks | |||||||||||
| 1 | LI | Central Africa 1 | 10 | 0.3 | 630 | 310 | 307 | 328 | 2,908 | 2,116 | 3,602 |
| 2 | LI | East Africa 1 | 11 | 0.3 | 61 | 108 | 98 | 108 | 1,580 | 423 | 1,585 |
| 3 | LI | East Africa 2 | 10 | 0.3 | -90 | 267 | 260 | 261 | 1,134 | 155 | 2,997 |
| 5 | LI | West Africa 1 | 11 | 0.3 | 450 | 418 | 409 | 420 | 1,419 | 191 | 1,885 |
| 32 | LI | North Pakistan and Afghanistan 1 | 11 | 0.3 | 461 | 522 | 523 | 523 | 96 | 496 | 181 |
| 34 | LMI | Rest of Pakistan 1 | 11 | 0.3 | 56 | 124 | 125 | 127 | 201 | 149 | 292 |
| 47 | LMI | India 1 | 13 | 0.3 | 0 | 0 | 0 | 0 | 477 | 86 | 585 |
| 48 | LMI | India 2 | 13 | 0.3 | 0 | 0 | 0 | 0 | 466 | 66 | 491 |
| High R0 (R0≥10) blocks | |||||||||||
| 49 | LMI | India 3 | 12 | 0.9 | 0 | 0 | 0 | 0 | 367 | 68 | 272 |
| 50 | LMI | India 4 | 11 | 0.9 | 0 | 3 | 2 | 3 | 276 | 49 | 272 |
| 51 | LMI | India 5 | 11 | 0.9 | 0 | 0 | 0 | 0 | 227 | 58 | 286 |
| 52 | LMI | India 6 | 11 | 0.9 | 0 | 0 | 0 | 0 | 223 | 57 | 226 |
| 53 | LMI | India 7 | 11 | 0.9 | 0 | 0 | 0 | 0 | 330 | 25 | 211 |
| 54 | LMI | India 8 | 11 | 0.9 | 0 | 0 | 0 | 0 | 299 | 31 | 283 |
| 55 | LMI | India 9 | 11 | 0.9 | 0 | 0 | 0 | 1 | 273 | 62 | 329 |
| 56 | LMI | India 10 | 11 | 0.9 | 0 | 5 | 7 | 6 | 314 | 67 | 267 |
| 57 | LMI | India 11 | 10 | 0.9 | 0 | 0 | 0 | 0 | 321 | 32 | 307 |
| 58 | LMI | India 12 | 10 | 0.9 | 0 | 0 | 0 | 0 | 201 | 64 | 180 |
| 68 | LMI | South Asia 1 | 12 | 0.6 | 0 | 0 | 0 | 0 | 124 | 0 | 206 |
| 69 | LMI | South Asia 2 | 13 | 0.6 | 0 | 2 | 0 | 0 | 118 | 20 | 141 |
| Low RI coverage (≤0.3) blocks | |||||||||||
| 7 | LMI | Middle-income Africa-Arabia 1 | 8 | 0.3 | 375 | 729 | 734 | 786 | 746 | 615 | 1,107 |
| 8 | LMI | North Nigeria 1 | 8 | 0.1 | 524 | 509 | 509 | 511 | 202 | 249 | 327 |
| 9 | LMI | West Africa 2 | 9 | 0.3 | 13 | 13 | 13 | 13 | 835 | 276 | 1,378 |
| 13 | LMI | South Asia 3 | 8 | 0.3 | 1 | 1 | 1 | 1 | 18 | 45 | 72 |
| 36 | UMI | Mid-east 2 | 7 | 0.05 | 74 | 73 | 73 | 73 | 105 | 83 | 101 |
| 38 | LMI | Eurasia 2 | 7 | 0.3 | 2 | 0 | 0 | 0 | 1 | 1 | 1 |
| Other blocks (not High R0 (≥10) or low RI coverage (≤0.3)) | |||||||||||
| <10 | >0.3 | 236 | 291 | 216 | 231 | 3,879 | 953 | 4,655 | |||
Notes:
Reported cases after OPV2 cessation (for the period May 1, 2016 to December 31, 2022 as of April 25, 2023,
Modeled expected cases for the same period as reported
Abbreviations: cVDPV2, type 2 circulating vaccine-derived polioviruses; IL, income level; mOPV2, type 2 monovalent OPV; nOPV2, type 2 novel OPV; OPV, type oral poliovirus vaccine; R0, basic reproduction number; RI, routine immunization
Figure 1(a) shows the distribution of the cases for the 100 stochastic iterations for each year in the time horizon of 2022–2026 for each of the scenarios using box and whisker plots. The results generally show increasing variability with time with the use of mOPV2 or worst nOPV2, because as transmission continues longer into the time horizon, the specific importation events that restart transmission in new blocks lead to more iterations with high-consequence importations. The best nOPV2 scenario shows the highest interquartile ranges of cases in 2023. This occurs due to the fact that the best nOPV2 scenario is not seeding any new outbreaks and ending the transmission that occurs in some, but not all outbreaks. For all vaccine scenarios, the tails of the distributions that correspond to case counts that exceed the interquartile range increase with time.
Figure 1.
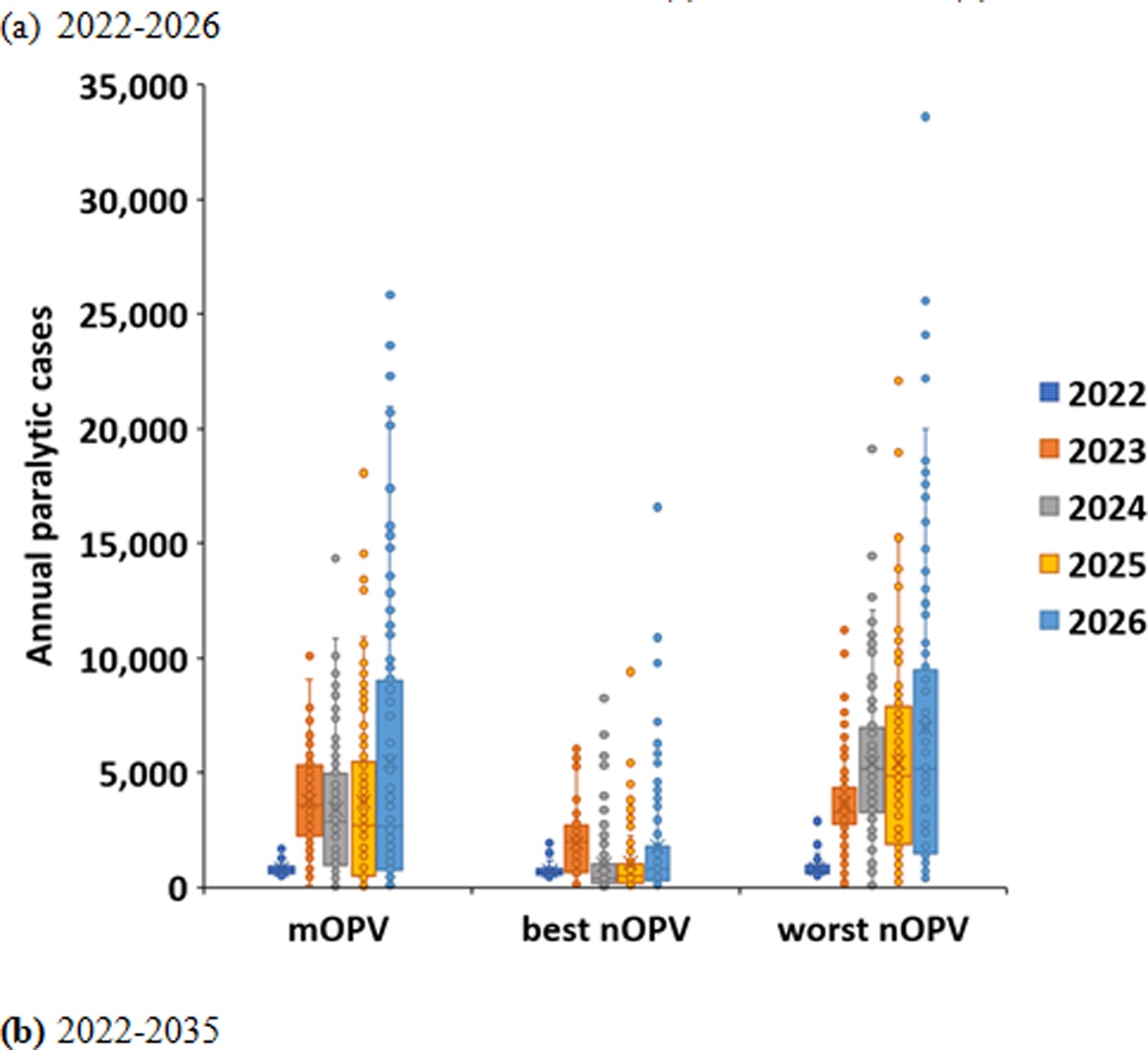
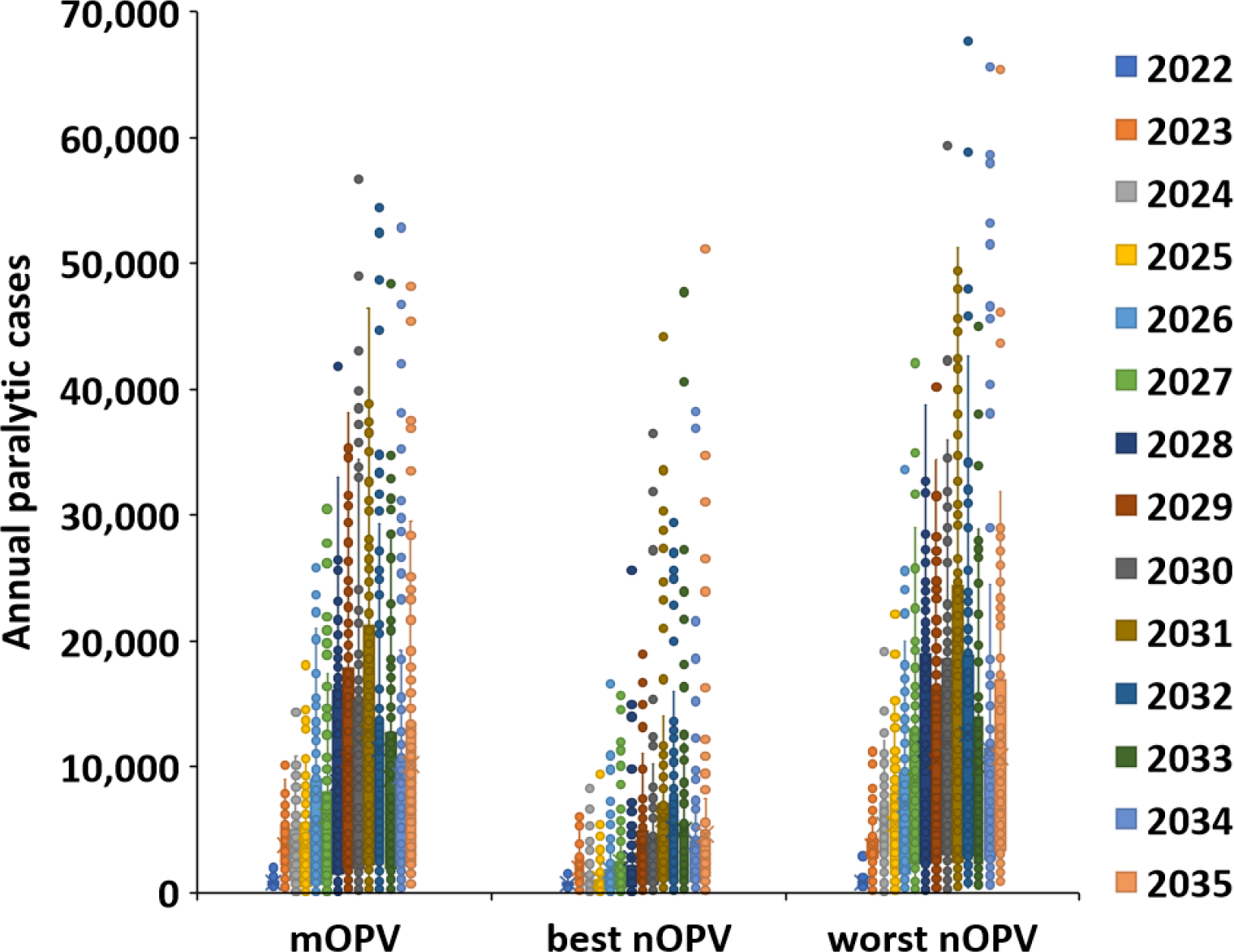
Variability among 100 stochastic iterations for RC with outbreak response using mOPV2, best nOPV2, or worst nOPV2 for (a) 2022–2026 and (b) 2022–2035
Since the global model includes subpopulations with properties that abstractly simulate the variability in conditions that influence poliovirus transmission potential in countries (e.g., different vaccine choices, coverage, levels of hygiene and sanitation, etc.), we cannot identify the specific countries that contribute the most to modeled prospective transmission. However, Table 1 lists the 26 abstractly modeled blocks with a high basic reproduction number (R0≥10) and/or blocks with low RI coverage subpopulations (RI coverage ≤0.3). Specifically, the top section lists the modeled blocks with both high R0 and low RI coverage subpopulations, the middle section lists the modeled blocks only with high R0, and the bottom section lists the modeled blocks with only low RI coverage subpopulations. These blocks and subpopulations represent high risk areas, in which live polioviruses can transmit most easily and fastest and/or cause the most cases.
The middle columns of Table 1 show the actual reported cVDPV2 cases since OPV2 cessation Yes (from May 2016 through December 31, 2022, using data as of April 25, 2023) and the modeled expected total cVDPV2 cases (without any adjustment for underreporting) for the same time period for comparison. Overall, these results show that 92% of the cVDPV2 cases reported since OPV2 cessation and 91–93% of expected cVDPV2 cases modeled for that period come from 11 out of 26 of these blocks in the model. With actual delays in reporting cases for some countries, Table 1 shows a few blocks for which the model estimates fall notably above or below the reported cases. For example, the ongoing outbreak in the Democratic Republic of the Congo (DRC) accounts for most of the reported cases for the Central Africa 1 block, with 360 confirmed cVDPV2 cases in 2022 reported as of April 25, 2023. With only 64 of the 360 cases reported when we performed the simulations in at the end of July of 2022, (and notably only 210 of the 360 cases reported by December 27, 2022, which provides context about the delays in reporting), the model fitting process reflected our understanding of the data and immunization plans at the time. Specifically, during the model updating process, the smaller epidemiological signal from retrospective data for the DRC led to input assumptions that produced fewer cases in 2022, and which also implied lower transmission in other countries within the same block. For blocks with relative overestimates of modeled cases compared to reported cases, the model assumptions (based on the epidemiological data available at the time) led to increased transmission in the block compared to confirmed cases (to date). Overall, the differences tend to cancel out, which implies small expected overall errors for a global trends and totals in the context of our abstract block and subpopulation model structure, which we reiterate does not specifically model or fit data to individual countries.
Table 2 lists the characteristics of the top 10 iterations (out of 100) with very high case counts for each mOPV2, best nOPV2, and worst nOPV2 for the time horizon of the current 2022–2026 GPEI Strategic Plan. The specific iteration numbers that appear in the top 10 differ between scenarios due to the stochastic and dynamic nature of the modeled exportations. However, the top 10 iterations in each scenario share the property of all importations and spread involving the 26 high-risk blocks and subpopulations described in Table 1.
Table 2.
Characteristics of the high case iterations (ordered by number of cDPV2 cases) for the period of 2022–2026 for
| (a) mOPV2 | |||||
|---|---|---|---|---|---|
| Iteration index | Number of cVDPV2 cases | Number of affected subpopulations | % cases in high R0 blocks* | % cases in low RI coverage blocks+ | % cases in blocks representing high transmission areas# |
| 23 | 44,125 | 197 | 80 | 36 | 54 |
| 55 | 43,054 | 182 | 71 | 49 | 40 |
| 80 | 41,443 | 180 | 75 | 42 | 56 |
| 5 | 39,823 | 173 | 81 | 46 | 56 |
| 93 | 38,616 | 159 | 77 | 43 | 51 |
| 45 | 38,191 | 177 | 75 | 40 | 53 |
| 87 | 37,616 | 124 | 58 | 57 | 16 |
| 81 | 35,092 | 131 | 77 | 43 | 52 |
| 33 | 34,485 | 153 | 87 | 44 | 65 |
| 57 | 34,481 | 124 | 62 | 44 | 46 |
| (b) best nOPV2 | |||||
| Iteration index | Number of cVDPV2 cases | Number of affected subpopulations | % cases in high R0 blocks* | % cases in low RI coverage blocks+ | % cases in blocks representing high transmission areas# |
| 6 | 26,209 | 119 | 75 | 32 | 50 |
| 9 | 25,119 | 101 | 95 | 70 | 43 |
| 100 | 23,682 | 103 | 65 | 61 | 35 |
| 3 | 23,222 | 100 | 87 | 52 | 76 |
| 66 | 17,634 | 73 | 76 | 45 | 50 |
| 5 | 16,860 | 74 | 68 | 84 | 0 |
| 64 | 14,947 | 78 | 53 | 67 | 0 |
| 98 | 14,278 | 90 | 26 | 29 | 3 |
| 94 | 12,944 | 52 | 61 | 91 | 4 |
| 38 | 12,585 | 69 | 44 | 64 | 0 |
| (c) worst nOPV2 | |||||
| Iteration index | Number of cVDPV2 cases | Number of affected subpopulations | % cases in high R0 blocks* | % cases in low RI coverage blocks+ | % cases in blocks representing high transmission areas# |
| 94 | 51,270 | 184 | 58 | 46 | 27 |
| 55 | 50,683 | 182 | 67 | 53 | 36 |
| 6 | 48,882 | 179 | 82 | 48 | 52 |
| 23 | 47,918 | 185 | 75 | 39 | 52 |
| 34 | 46,464 | 152 | 60 | 60 | 23 |
| 66 | 45,035 | 160 | 72 | 47 | 48 |
| 84 | 44,178 | 144 | 59 | 54 | 20 |
| 33 | 43,770 | 172 | 76 | 35 | 67 |
| 100 | 43,390 | 132 | 79 | 60 | 43 |
| 9 | 41,759 | 128 | 84 | 55 | 51 |
Notes:
R0≥10;
RI coverage ≤0.3,
blocks 47–58, 68–69 indicated in Table 1
Abbreviations: cVDPV2, type 2 circulating vaccine-derived polioviruses; type 2 monovalent OPV; nOPV2, type 2 novel OPV; OPV, oral poliovirus vaccine; R0, basic reproduction number; RI, routine immunization
For each of the top 10 iterations listed in Table 2, Figure 2 shows the corresponding modeled total cases by year (solid lines) compared to the expected value of modeled cVDPV2 cases based on all 100 stochastic iterations (dashed lines) for the time horizon of the current 2022–2026 GPEI Strategic Plan. The number of cases in each year reflects the path that the importations take that lead to outbreaks in different subpopulations, with the timing of the importations into the blocks listed in Table 1 accounting for the peaks in Figure 2 due to their higher transmission potential and/or lower coverage. Specifically, once cVDPV2 enters the high R0 blocks that represent conditions like India and Bangladesh (see last column of Table 2), the transmission spreads extensively such that control would require over 1 billion doses of filled OPV in the stockpile plus plans and resources to rapidly conduct oSIAs to administer the doses, which is beyond the supply capability of the system in the 2022–2026 time horizon. These iterations show a rapid increase in case count, where the high R0 blocks account for up to 67% of all estimated cases. Earlier modeling observations of this type of behavior motivated pre-OPV2 cessation modeling to the include OPV restart in those studies (Duintjer Tebbens et al., 2015; Kalkowska, Pallansch, Cochi, et al., 2021). We observe this behavior for the mOPV2 or worst nOPV2 scenarios within the short time horizon (2022–26).
Figure 2.
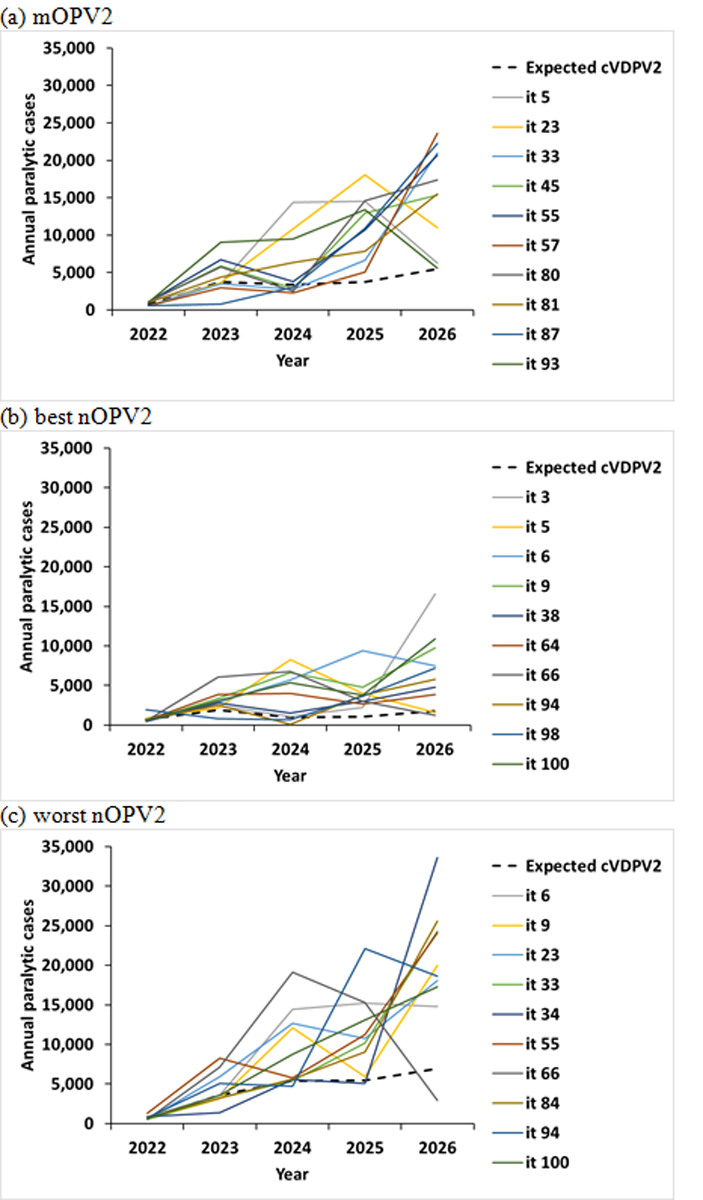
10 (out of 100) worst performing stochastic iterations for RC with outbreak response for the 2022–2026 time horizon using (a) mOPV2, (b) best nOPV2, or (c) worst nOPV2
For the extended time horizon of 2022–2035, Figure 1(b) shows the distribution of the cases for the 100 stochastic iterations for each year, and Table 3 and Figure 3 show the comparable results to those reported in Table 2 and Figure 2. As shown by the doubling of the y-axis scale for Figure 1(b) compared to Figure 1(a) and the results reported in Table 3, the number of overall expected cases increases with the extended time horizon. As expected, the specific iterations in the top 10% of the case counts depend on the timing of when the importations reach the high transmission settings in Table 1. Similar to Figure 2, the model reaches peaks of cases in Figure 3 when the cVDPV2 enters the high transmission settings (i.e., blocks representing conditions like India and Bangladesh summarized in right column of Table 3). Given the extended time horizon, the peaks become more easily observable for the best nOPV2 scenario compared to short term use of best nOPV2 in Figure 2. The best nOPV2 scenario requires the extended time horizon to show these effects because the assumptions for nOPV2 for this scenario reduce the number of importations, and therefore it takes longer for imported outbreak viruses to reach the high R0 blocks representing conditions like India and Bangladesh.
Table 3.
Characteristics of the high case iterations (ordered by number of cDPV2 cases) for the period of 2022–2035 for
| (a) mOPV2 | |||||
|---|---|---|---|---|---|
| Iteration index | Number of cVDPV2 cases | Number of affected subpopulations | % cases in high R0 blocks* | % cases in low RI coverage blocks+ | % cases in blocks representing high transmission areas# |
| 37 | 169,097 | 422 | 46 | 40 | 29 |
| 15 | 157,579 | 413 | 49 | 41 | 31 |
| 30 | 155,526 | 388 | 52 | 38 | 37 |
| 95 | 148,077 | 378 | 51 | 35 | 33 |
| 53 | 147,021 | 325 | 58 | 48 | 36 |
| 52 | 145,936 | 310 | 55 | 46 | 34 |
| 100 | 142,682 | 395 | 48 | 41 | 30 |
| 62 | 142,629 | 362 | 56 | 47 | 38 |
| 22 | 142,520 | 398 | 51 | 40 | 29 |
| 13 | 140,721 | 372 | 56 | 40 | 39 |
| (b) best nOPV2 | |||||
| Iteration index | Number of cVDPV2 cases | Number of affected subpopulations | % cases in high R0 blocks* | % cases in low RI coverage blocks+ | % cases in blocks representing high transmission areas# |
| 8 | 127,392 | 397 | 51 | 43 | 34 |
| 58 | 124,342 | 355 | 57 | 38 | 42 |
| 18 | 114,699 | 311 | 66 | 25 | 58 |
| 56 | 109,374 | 295 | 58 | 35 | 46 |
| 62 | 107,518 | 352 | 44 | 34 | 34 |
| 72 | 107,213 | 318 | 67 | 47 | 44 |
| 26 | 97,413 | 243 | 57 | 39 | 42 |
| 86 | 96,213 | 263 | 77 | 36 | 64 |
| 37 | 94,151 | 277 | 67 | 39 | 47 |
| 6 | 86,348 | 330 | 49 | 32 | 31 |
| (c) worst nOPV2 | |||||
| Iteration index | Number of cVDPV2 cases | Number of affected subpopulations | % cases in high R0 blocks* | % cases in low RI coverage blocks+ | % cases in blocks representing high transmission areas# |
| 16 | 191,122 | 442 | 53 | 38 | 35 |
| 15 | 188,336 | 457 | 52 | 41 | 34 |
| 73 | 185,292 | 418 | 54 | 40 | 35 |
| 74 | 181,006 | 438 | 49 | 40 | 29 |
| 13 | 179,567 | 454 | 52 | 40 | 33 |
| 21 | 179,088 | 409 | 56 | 41 | 35 |
| 8 | 177,033 | 428 | 51 | 41 | 32 |
| 24 | 174,137 | 443 | 51 | 40 | 32 |
| 31 | 170,745 | 419 | 53 | 36 | 36 |
| 22 | 168,351 | 371 | 54 | 36 | 37 |
Notes:
R0≥10;
RI coverage ≤0.3,
blocks 47–58, 68–69 indicated in Table 1
Abbreviations: cVDPV2, type 2 circulating vaccine-derived polioviruses; type 2 monovalent OPV; nOPV2, type 2 novel OPV; OPV, oral poliovirus vaccine; R0, basic reproduction number; RI, routine immunization
Figure 3.
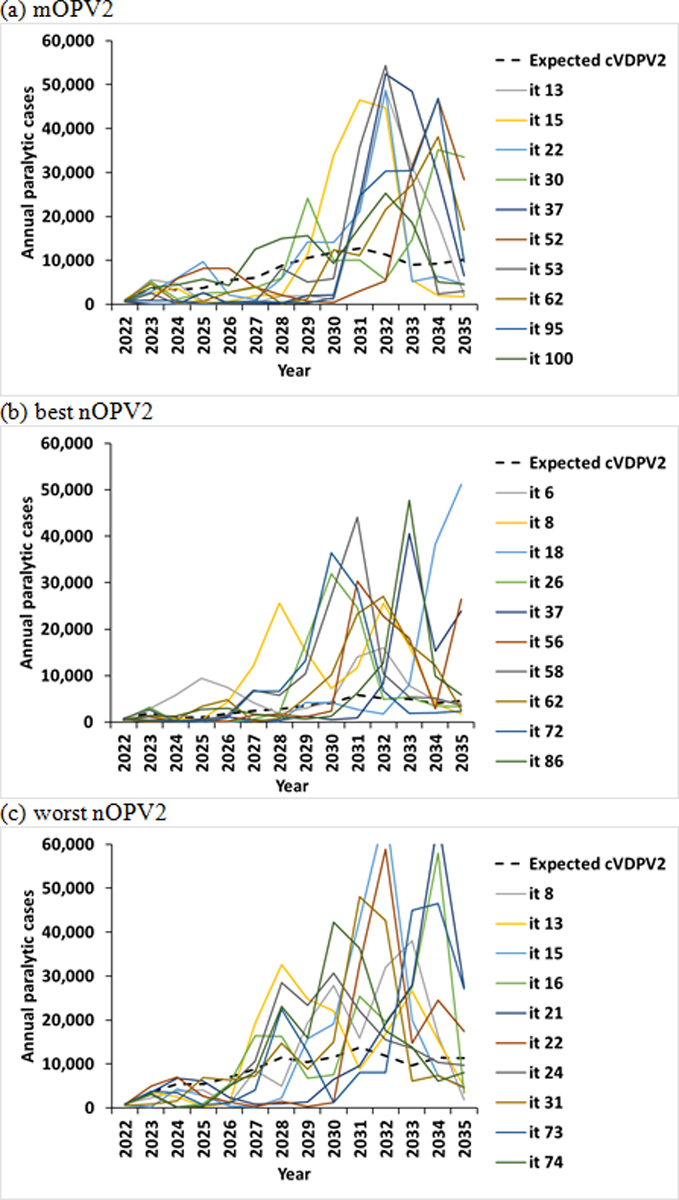
10 (out of 100) worst performing stochastic iterations for RC with outbreak response for 2022–2035 time horizon using (a) mOPV2, (b) best nOPV2, or (c) worst nOPV2
4. Discussion
The probability of successfully stopping type 2 transmission only with outbreak response campaigns of the current quality continues to decline (Kalkowska, Pallansch, Cochi, et al., 2021; Kalkowska, Pallansch, Wassilak, et al., 2021; Kalkowska, Pallansch, Wilkinson, et al., 2021; Kalkowska, Wassilak, Pallansch, et al., 2023). Despite an estimated 6% chance of needing to restart OPV2 prior to OPV2 cessation (Duintjer Tebbens et al., 2015), the probability of successful OPV2 cessation continued to drop since 2017 (Kalkowska, Pallansch, Cochi, et al., 2021; Kalkowska, Pallansch, Wassilak, et al., 2021; Kalkowska, Pallansch, Wilkinson, et al., 2021; Kalkowska, Wassilak, Pallansch, et al., 2023).
Eradication represents an unforgiving goal (Thompson & Duintjer Tebbens, 2017), which requires ending all transmission in all areas contemporaneously. Some earlier discussions of the challenges of eradication focused on the weak links (Barrett, 2003; Barrett, 2009; Barrett & Hoel, 2007), which modeling studies previously referred to as “under-vaccinated subpopulations” (Kalkowska et al., 2015; Kalkowska et al., 2014a, 2014b, 2018) and others currently refer to as populations in “critical” or “consequential” geographies (Independent Monitoring Board of the Global Polio Eradication Initiative, 2022). As time increases since the OPV2 cessation, many countries now include large birth cohorts that have not been exposed to OPV2-related viruses since 2016. The increasing vulnerability of populations to transmission (Duintjer Tebbens, Hampton, & Thompson, 2016a, 2016b; Duintjer Tebbens, Hampton, et al., 2018; Duintjer Tebbens, Hampton, Wassilak, et al., 2016) following the importation of type 2 polioviruses means that responding to outbreaks in these countries will require very large outbreak response activities involving age groups expanded beyond the <5-year-old age range. Our results show that in the absence of more timely, larger, and better outbreak response, and without a concerted effort to raise the intestinal immunity to type 2 polioviruses in high-risk countries, there is no probability of die out of cVDPV2s and a risk of uncontrolled type 2 outbreaks, regardless of vaccine choice. This may require reintroduction of an OPV2 in RI, followed by re-coordination of the cessation of all OPV2 use to achieve cessation of all transmission of type 2 polioviruses.
The results of this analysis come with several limitations. In particular, the model uses conceptual characterization of global variability using block/subpopulation structure and the simplified modeling approach used to simulate effective poliovirus introductions during exportation to new blocks/subpopulations. This simplification allows for faster simulation times but does not allow for direct comparisons of specific blocks with specific countries. Moreover, the results depend on available information/assumptions about the initial conditions as of the end of 2021, expected future policies/actions, the uncertain properties of nOPV2, the uncertain global political climate affecting outbreak response activities, and the implicit assumption of unlimited vaccine supplies.
As type 2 transmission continues, our results suggest that the chances of effectively controlling type 2 poliovirus outbreaks continue to decline. In the model, if any OPV2-related virus reaches high transmission settings, like some areas of India and Bangladesh, very high type 2 case counts would likely follow. Recent importations and transmission of cVDPV2s in high-income countries, including Israel (Zuckerman et al., 2022), the UK (United Kingdom Department of Health & Social Care, 2022), and the US (Link-Gelles et al., 2022; Ryerson et al., 2022), confirm that cVDPV transmission can occur even in countries with high overall reported IPV immunization coverage in communities with low coverage (Thompson et al., 2012), with paralytic cases possible in these communities. Countries with relatively lower immunization coverage should expect to fair worse with respect to potential case counts, and they should recognize the need for large and high-quality outbreak responses if they want to keep case counts lower. However, insufficient quantities of vaccine available for responding to type 2 outbreaks may limit the ability of countries to respond, as occurred in the past, and in this regard, the situation could prove more challenging than what we modeled. These insights may lead to further discussions about the need to improve the quality of cVDPV2 outbreak response, including changes in strategy and tactics that make responses more timely, larger, and higher quality (Kalkowska, Wassilak, Pallansch, et al., 2023). Discussions could also begin to consider the appropriate triggers to preemptively restart OPV2 use in RI and potentially in preventive SIAs in low coverage setting in some OPV-using countries.
Funding
The first and last two authors acknowledge support for this publication under Cooperative Agreement Number NU2RGH001915-02-00 funded by the Centers for Disease Control and Prevention. The views expressed are solely those of the authors and do not necessarily represent the official views of the US Centers for Disease Control and Prevention or Department of Health and Human Services.
References
- Barrett S (2003). Global disease eradication. J Europ Econ Assoc, 1(2/3), 591–600. [Google Scholar]
- Barrett S (2009). Polio eradication: Strengthening the weakest links. Health Aff, 28(4), 1079–1090. [DOI] [PubMed] [Google Scholar]
- Barrett S, & Hoel M (2007). Optimal disease eradication. Environment and Development Economics, 12(5), 627–652. [Google Scholar]
- Duintjer Tebbens RJ, Hampton LM, & Thompson KM (2016a). Implementation of coordinated global serotype 2 oral poliovirus vaccine cessation: Risks of inadvertent trivalent oral poliovirus vaccine use. BMC Infectious Diseases, 16, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Hampton LM, & Thompson KM (2016b). Implementation of coordinated global serotype 2 oral poliovirus vaccine cessation: Risks of potential non-synchronous cessation. BMC Infect Dis, 16, 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Hampton LM, & Thompson KM (2018). Planning for globally coordinated cessation of bivalent oral poliovirus vaccine: risks of non-synchronous cessation and unauthorized oral poliovirus vaccine use. BMC Infect Dis, 18(1), 165. 10.1186/s12879-018-3074-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Hampton LM, Wassilak SGF, Pallansch MA, Cochi SL, & Thompson KM (2016). Maintenance and intensification of bivalent oral poliovirus vaccine use prior to its coordinated global cessation. J Vaccines Vaccinat, 7(5), 340. 10.4172/2157-7560.1000340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Kalkowska DA, & Thompson KM (2018). Poliovirus containment risks and their management. Future Virol, 13(9), 617–628. 10.2217/fvl-2018-0079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Pallansch MA, Kew OM, Cáceres VM, Jafari H, Cochi SL, Aylward RB, & Thompson KM (2006). Risks of paralytic disease due to wild or vaccine-derived poliovirus after eradication. Risk Anal, 26(6), 1471–1505. [DOI] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Pallansch MA, Wassalik SGF, Cochi SL, & Thompson KM (2015). An economic analysis of poliovirus risk management policy options for 2013–2052. BMC Infect Dis, 15(389), doi: 10.1186/s12879-12015-11112-12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Pallansch MA, Wassilak SGF, Cochi SL, & Thompson KM (2016). Characterization of outbreak response strategies and potential vaccine stockpile needs for the polio endgame. BMC Infecti Dis, 16, 137. 10.1186/s12879-016-1465-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, & Thompson KM (2018). Polio endgame risks and the possibility of restarting the use of oral poliovirus vaccine. Expert Rev Vaccines, 17(8), 739–751. 10.1080/14760584.2018.1506333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Polio Eradication Initiative. (2013). Polio eradication and endgame Strategic Plan (2013–2018). World Health Organization. Retrieved Jun 4 2019 from http://polioeradication.org/wp-content/uploads/2016/07/PEESP_EN_A4.pdf [Google Scholar]
- Global Polio Eradication Initiative. (2016). Standard operating procedures: Responding to a poliovirus event or outbreak: Part 1: General SOPs. World Health Organization. Retrieved April 20 2016 from https://s3.amazonaws.com/gpei-tk/reference_links/en/GPEI_SOPs.pdf [Google Scholar]
- Global Polio Eradication Initiative. (2019). Polio eradication and endgame strategic plan (2019–2023). World Health Organization. Retrieved Jun 4 2019 from https://polioeradication.org/wp-content/uploads/2019/06/english-polio-endgame-strategy.pdf [Google Scholar]
- Global Polio Eradication Initiative. (2020). Strategy for the response to type 2 circulating vaccine-derived poliovirus 2020–2021: Addendum to the Polio eradication and endgame strategic plan (2019–2023). World Health Organization. Retrieved Mar 10 2020 from http://polioeradication.org/wp-content/uploads/2020/04/Strategy-for-the-response-to-type-2-circulating-Vaccine-Derived-Poliovirus-20200406.pdf [Google Scholar]
- Global Polio Eradication Initiative. (2021). Polio eradication strategy 2022–2026: Delivering on a promise. World Health Organization. Retrieved Jun 11 2021 from https://polioeradication.org/wp-content/uploads/2021/06/polio-eradication-strategy-2022-2026-pre-publication-version-20210609.pdf [Google Scholar]
- Global Polio Eradication Initiative. (2022). nOPV2: Clinical development summary updated June 2022. World Health Organization. Retrieved 11 Aug 2022 from https://polioeradication.org/wp-content/uploads/2022/06/nOPV2-Clinical-Development-Summary_June-2022-Update_Final-EN.pdf [Google Scholar]
- Global Polio Eradication Initiative. (2023a). Global circulating vaccine-derived poliovirus (cVDPV) as of 25 April 2023. World Health Organization. Retrieved 2 May 2023 from https://polioeradication.org/wp-content/uploads/2023/04/weekly-polio-analyses-cVDPV-20230425.pdf [Google Scholar]
- Global Polio Eradication Initiative. (2023b). GPEI Statement on cVDPV2 detections in Burundi and Democratic Republic of the Congo. World Health Organization. Retrieved 25 April 2023 from https://polioeradication.org/news-post/gpei-statement-on-cvdpv2-detections-in-burundi-and-democratic-republic-of-the-congo/ [Google Scholar]
- Hampton LM, Farrell M, Ramirez-Gonzalez A, Menning L, Shendale S, Lewis I, Rubin J, Garon J, Harris J, Hyde T, Wassilak SGF, Patel M, Nandy R, Chang-Blanc D, & Immunization Systems Management Group of the Global Polio Eradication Initiative. (2016). Cessation of trivalent oral poliovirus vaccine and introduction of inactivated poliovirus vaccine - Worldwide, 2016. Morb Mortal Wkly Rep, 65(35), 934–938. 10.15585/mmwr.mm6535a3 [DOI] [PubMed] [Google Scholar]
- Independent Monitoring Board of the Global Polio Eradication Initiative. (2022). Highs and lows in the quest for zero, 21st Report, April 2022. Retrieved Aug 24 from https://polioeradication.org/wp-content/uploads/2022/05/21st-IMB-report-20220430.pdf
- Kalkowska DA, Badizadegan K, & Thompson KM (2023). Outbreak management strategies for cocirculation of multiple poliovirus types. Vaccine 10.1016/j.vaccine.2023.04.037 [DOI] [PubMed] [Google Scholar]
- Kalkowska DA, Duintjer Tebbens RJ, Grotto I, Shulman LM, Anis E, Wassilak SGF, Pallansch MA, & Thompson KM (2015). Modeling options to manage type 1 wild poliovirus imported into Israel in 2013. J Infect Dis, 211(11), 1800–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkowska DA, Duintjer Tebbens RJ, & Thompson KM (2014a). Modeling strategies to increase population immunity and prevent poliovirus transmission in the high-risk area of northwest Nigeria. J Infect Dis, 210(Suppl 1), S412–S423. [DOI] [PubMed] [Google Scholar]
- Kalkowska DA, Duintjer Tebbens RJ, & Thompson KM (2014b). Modeling strategies to increase population immunity and prevent poliovirus transmission in two high-risk areas in northern India. J Infect Dis, 210(Suppl 1), S398–S411. [DOI] [PubMed] [Google Scholar]
- Kalkowska DA, Duintjer Tebbens RJ, & Thompson KM (2018). Another look at silent circulation of poliovirus in small populations. Infect Dis Model, 3, 107–117. 10.1016/j.idm.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkowska DA, Pallansch MA, Cochi SL, Kovacs SD, Wassilak SGF, & Thompson KM (2021). Updated characterization of post-OPV cessation risks: Lessons from 2019 serotype 2 outbreaks and implications for the probability of OPV restart. Risk Anal, 41(2), 320–328. 10.1111/risa.13555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkowska DA, Pallansch MA, & Thompson KM (2019). Updated modelling of the prevalence of immunodeficiency-associated long-term vaccine-derived poliovirus (iVDPV) excreters. Epidemiology and Infection, 147, e295, Article e295. 10.1017/S095026881900181X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkowska DA, Pallansch MA, Wassilak SGF, Cochi SL, & Thompson KM (2021). Global transmission of live polioviruses: Updated integrated dynamic modeling of the polio endgame. Risk Anal, 41(2), 248–265. 10.1111/risa.13447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkowska DA, Pallansch MA, Wassilak SGF, Cochi SL, & Thompson KM (2023). Serotype 2 oral poliovirus vaccine (OPV2) choices and the consequences of delaying outbreak response. Vaccine, 41 Suppl 1, A136–A141. 10.1016/j.vaccine.2021.04.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkowska DA, Pallansch MA, Wilkinson A, Bandyopadhyay AS, Konopka-Anstadt JL, Burns CC, Oberste MS, Wassilak SGF, Badizadegan K, & Thompson KM (2021). Updated characterization of poliovirus outbreak response strategies for 2019–2029: Impacts of the use of novel OPV2 strains. Risk Anal, 41(2), 329–348. 10.1111/risa.13622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkowska DA, Voorman A, Pallansch MA, Wassilak SGF, Cochi SL, Badizadegan K, & Thompson KM (2023). The impact of disruptions caused by the COVID-19 pandemic on global polio eradication. Vaccine, 41 Suppl 1, A12–A18. 10.1016/j.vaccine.2021.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkowska DA, Wassilak SGF, Pallansch MA, Burns CC, Wiesen E, Durry E, Badizadegan K, & Thompson KM (2023). Outbreak response strategies with type 2-containing oral poliovirus vaccines. Vaccine, 41 Suppl 1, A142–A152. 10.1016/j.vaccine.2022.10.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkowska DA, Wassilak SGF, Wiesen E, Burns CC, Pallansch MA, Badizadegan K, & Thompson KM (2023). Coordinated global cessation of oral poliovirus vaccine use: Options and potential consequences. Risk Anal, (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link-Gelles R, Lutterloh E, Schnabel Ruppert P, Backenson PB, St George K, Rosenberg ES, Anderson BJ, Fuschino M, Popowich M, Punjabi C, Souto M, McKay K, Rulli S, Insaf T, Hill D, Kumar J, Gelman I, Jorba J, Ng TFF, . . . Routh J (2022). Public Health Response to a Case of Paralytic Poliomyelitis in an Unvaccinated Person and Detection of Poliovirus in Wastewater - New York, June-August 2022. Morb Mortal Wkly Rep, 71(33), 1065–1068. 10.15585/mmwr.mm7133e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklin GR, Peak C, Eisenhawer M, Kurji F, Mach O, Konz J, Gast C, Bachtiar NS, Bandyopadhyay AS, Zipursky S, & n OPVWG (2023). Enabling accelerated vaccine roll-out for Public Health Emergencies of International Concern (PHEICs): Novel Oral Polio Vaccine type 2 (nOPV2) experience. Vaccine, 41 Suppl 1(Suppl 1), A122–A127. 10.1016/j.vaccine.2022.02.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Burns CC, Jorba J, Shulman LM, Macadam A, Klapsa D, Majumdar M, Bullows J, Frolov A, Mate R, Bujaki E, Castro CJ, Bullard K, Konz J, Hawes K, Gauld J, Blake IM, Mercer LD, Kurji F, . . . Zipursky S (2022). Genetic characterization of novel oral polio vaccine type 2 viruses during initial use phase under emergency use listing - Worldwide, March-October 2021. Morb Mortal Wkly Rep, 71(24), 786–790. 10.15585/mmwr.mm7124a2 [DOI] [PubMed] [Google Scholar]
- Rachlin A, Patel JC, Burns CC, Jorba J, Tallis G, O’Leary A, Wassilak SGF, & Vertefeuille JF (2022). Progress toward polio eradication - worldwide, January 2020-April 2022. Morb Mortal Wkly Rep, 71(19), 650–655. 10.15585/mmwr.mm7119a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryerson AB, Lang D, Alazawi MA, Neyra M, Hill DT, St George K, Fuschino M, Lutterloh E, Backenson B, Rulli S, Ruppert PS, Lawler J, McGraw N, Knecht A, Gelman I, Zucker JR, Omoregie E, Kidd S, Sugerman DE, . . . Rosenberg ES (2022). Wastewater Testing and Detection of Poliovirus Type 2 Genetically Linked to Virus Isolated from a Paralytic Polio Case - New York, March 9-October 11, 2022. Morb Mortal Wkly Rep, 71(44), 1418–1424. 10.15585/mmwr.mm7144e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KM (2022a). Effectiveness of a new vaccine for outbreak response and the increasingly complicated polio endgame. Lancet Glob Health, 10(12), e1697–e1698. 10.1016/S2214-109X(22)00452-1 [DOI] [PubMed] [Google Scholar]
- Thompson KM (2022b). Polio eradication: what kind of world do we want? Lancet Infect Dis, 22(2), 161–163. 10.1016/s1473-3099(21)00458-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KM, & Duintjer Tebbens RJ (2017). Lessons from the polio endgame: Overcoming the failure to vaccinate and the role of subpopulations in maintaining transmission. J Infect Dis, 216(suppl_1), S176–s182. 10.1093/infdis/jix108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KM, Duintjer Tebbens RJ, & Pallansch MA (2006). Evaluation of response scenarios to potential polio outbreaks using mathematical models. Risk Anal, 26(6), 1541–1556. 10.1111/j.1539-6924.2006.00843.x [DOI] [PubMed] [Google Scholar]
- Thompson KM, & Kalkowska DA (2020). Review of poliovirus modeling performed from 2000–2019 to support global polio eradication. Expert Rev Vaccines, 19(7), 661–686. 10.1080/14760584.2020.1791093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KM, & Kalkowska DA (2021). Reflections on modeling poliovirus transmission and the polio eradication endgame. Risk Anal, 41(2), 229–247. 10.1111/risa.13484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KM, Kalkowska DA, & Badizadegan K (2022). Health economic analysis of vaccine options for the polio eradication endgame: 2022–2036. Expert Rev Vaccines, 1–8. 10.1080/14760584.2022.2128108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KM, Kalkowska DA, & Badizadegan K (2023). Looking back at prospective modeling of outbreak response strategies for managing global type 2 oral poliovirus vaccine (OPV2) cessation [Original Research]. Front Public Health, 11. 10.3389/fpubh.2023.1098419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KM, Wallace GS, Duintjer Tebbens RJ, Smith PH, Barskey AE, Pallansch MA, Gallagher KM, Alexander JP, Armstrong GL, Cochi SL, & Wassilak SGF (2012). Trends in the risk of U.S. polio outbreaks and poliovirus vaccine availability for response. Public Health Rep, 127(1), 23–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Kingdom Department of Health & Social Care. (2022). Joint Committee on Vaccination and Immunisation statement on vaccination strategy for the ongoing polio incident. Retrieved Aug 24 from https://www.gov.uk/government/publications/vaccination-strategy-for-ongoing-polio-incident-jcvi-statement/joint-committee-on-vaccination-and-immunisation-statement-on-vaccination-strategy-for-the-ongoing-polio-incident
- World Health Assembly. (2008). Poliomyelitis: mechanism for management of potential risks to eradication (resolution 61.1), WHO, Geneva, 19–24 May 2008. World Health Organization. Retrieved Jun 4 from http://apps.who.int/gb/ebwha/pdf_files/WHA61-REC1/A61_Rec1-part2-en.pdf [Google Scholar]
- World Health Organization. (2010). Global Polio Eradication Initiative - Strategic Plan 2010–2012. Retrieved Sep 6 from http://www.polioeradication.org/Portals/0/Document/StrategicPlan/StratPlan2010_2012_ENG.pdf
- World Health Organization. (2022). GACVS (Global Advisory Committee of Vaccine Safety) Sub-Committee on nOPV2 Safety June 30, 2022 – Virtual Meeting Summary Note for the Record. Retrieved Aug 24 from https://polioeradication.org/wp-content/uploads/2022/08/GACVS-sub-committee-on-novel-opv2-safety.pdf
- World Health Organization. (2023). Report of the second joint meeting (hybrid) of the WHO Global Advisory Committee on Vaccine Safety and the WHO Advisory Committee on Safety of Medicinal Products, 14–16 December 2022. Wkly Epidemiol Rec, 98(09), 83–92. https://apps.who.int/iris/handle/10665/366343 [Google Scholar]
- Zuckerman NS, Bar-Or I, Sofer D, Bucris E, Morad H, Shulman LM, Levi N, Weiss L, Aguvaev I, Cohen Z, Kestin K, Vasserman R, Elul M, Fratty IS, Geva M, Wax M, Erster O, Yishai R, Hecht-Sagie L, . . . Weil M (2022). Emergence of genetically linked vaccine-originated poliovirus type 2 in the absence of oral polio vaccine, Jerusalem, April to July 2022. Euro Surveill, 27(37). 10.2807/1560-7917.Es.2022.27.37.2200694 [DOI] [PMC free article] [PubMed] [Google Scholar]


