Abstract
Due to the very low, but non-zero, paralysis risks associated with the use of oral poliovirus vaccine (OPV), eradicating poliomyelitis requires ending all OPV use globally. The Global Polio Eradication Initiative (GPEI) coordinated cessation of Sabin type 2 OPV (OPV2 cessation) in 2016, except for emergency outbreak response. However, as of early 2023, plans for cessation of bivalent OPV (bOPV, containing types 1 and 3 OPV) remain undefined, and OPV2 use for outbreak response continues due to ongoing transmission of type 2 polioviruses and reported type 2 cases. Recent development and use of a genetically stabilized novel type 2 OPV vaccine (nOPV2) leads to additional potential vaccine options and increasing complexity in strategies for the polio endgame. Prior applications of integrated global risk, economic, and poliovirus transmission modeling consistent with GPEI strategic plans that preceded OPV2 cessation explored OPV cessation dynamics and the evaluation of options to support globally coordinated risk management efforts. The 2022–2026 GPEI strategic plan highlighted the need for early bOPV cessation planning. We review published modeling and explore bOPV cessation immunization options as of 2022, assuming that the GPEI partners will not support restart of the use of any OPV type in routine immunization after a globally-coordinated cessation of such use. We model the potential consequences of globally-coordinating bOPV cessation in 2027, as anticipated in the 2022–2026 GPEI strategic plan. We do not find any options for bOPV cessation likely to succeed without a strategy of bOPV intensification to increase population immunity prior to cessation.
Keywords: polio, eradication, dynamic modeling, oral poliovirus vaccine
1. Introduction
As the initial target year of 2000 for polio eradication approached, key partners of the Global Polio Eradication Initiative (GPEI) discussed ending the use of oral poliovirus vaccine (OPV) as part of the polio endgame after WPV eradication [1]. OPV use comes with rare, but non-zero risks of vaccine associated paralytic polio (VAPP) and vaccine-derived polioviruses (VDPVs) [2]. Some early proponents of OPV cessation continue to suggest urgency [3, 4], while others argue that OPV use should continue indefinitely and GPEI should abandon the objective of eradication of all poliovirus transmission [5, 6]. Recently, some studies supported continued use of OPV due to potential benefits of secondary vaccine effects [7–9], although other studies highlighted the uncertainty about these effects and any associated health-economic implications [10–12].
Over the last 2 decades, our integrated global risk, economic, and poliovirus transmission modeling explored numerous aspects of the polio endgame, with vaccine choices representing a primary focus [13]. These studies included the identification and quantification of OPV-related risks [2], support for the case for globally coordinated OPV cessation [14, 15], development of outbreak response protocols [16], and stockpile creation to manage polio endgame risks prior to OPV cessation [15]. Following a 2008 resolution by the World Health Assembly related to OPV cessation [17], studies in the early 2010s included discussion of polio vaccine options for the endgame and development of prerequisites for successful OPV cessation [18]. Further modeling studies identified risk management strategies to increase the probability of successful OPV cessation [19–22]. These studies used available information about routine immunization (RI) and supplemental immunization activities (SIAs) and focused on identifying opportunities to prevent the need for outbreak response after cessation, with emphasis on OPV intensification prior to cessation [21]. One analysis also explored the role of inactivated poliovirus vaccine (IPV) in RI and highlighted its expected inability to stop outbreaks, particularly in the places most likely to experience outbreaks [22].
OPV cessation as a polio endgame strategy comes with costs and risks. If countries and the GPEI partners used the model-recommended risk management strategies for the polio endgame, 2015 model estimates (prior to OPV2 cessation) suggested an approximately 6% chance of needing to restart the production of OPV2 vaccine and its use in RI to respond to significant levels of type 2 poliovirus transmission after OPV2 cessation [19]. A 2016 study emphasized the importance of aggressive outbreak response after OPV2 cessation to rapidly shut down any outbreaks and manage risks [20]. Recognition of these risks led to the GPEI to develop standard operating procedures for outbreak response SIAs (oSIAs) [23] and a stockpile of Sabin type 2 monovalent OPV (mOPV2) for oSIAs. Prior to OPV2 cessation, integrated modeling studies demonstrated the importance of shutting down all identified type 2 transmission completely before and as soon as possible after (if any occurred) homotypic OPV cessation [24, 25]. These studies used updated information about RI and SIAs to demonstrate the expected decreases in population immunity following OPV2 cessation as a function of time after OPV2 cessation and the anticipated increases in the vulnerability of populations to restarting transmission of type 2 polioviruses following importations of circulating vaccine derived poliovirus type 2 (cVDPV2) and/or mOPV2 used in oSIAs [24, 25].
After OPV2 cessation occurred, integrated modeling studies that reported on the status of OPV2 cessation as of end-2016 [26], early 2018 [27], end of 2019 [28], early 2020 [29], and late 2021 [30] focused on the need for improvements in implementing oSIAs and highlighted that the polio endgame was off-track. Continued deficiencies in the promptness, scope, and/or quality of mOPV2 oSIAs continue to exacerbate the situation in many settings [31]. After updating the characterization of OPV2 cessation risks based on immunization and epidemiological experience through early 2020, updated model estimates of the probability of OPV2 restart increased to over 80% [29]. A 2021 reflection [32] on the OPV2 experience compared the (i) then-prospective assumptions about GPEI strategies and performance used in modeling to support the 2013–2018 GPEI strategic plan [33] prior to OPV2 cessation, with the (ii) polio endgame path actually observed through 2020. That reflection identified model assumptions that required updating [32] prior to conducting further prospective polio endgame modeling, including the observation of worse oSIA performance than required for success in pre-cessation modeling [28, 29, 34–36]. A recent look back analysis quantified the probability of successful OPV2 cessation if the modelers knew then what we know now about OPV2 cessation risks and post-OPV2 cessation oSIA characteristics [37]. As of early-2023, type 2 transmission continues [38].
The development and deployment of a genetically modified novel type 2 OPV (nOPV2), which designers engineered for increased genetic stability and lower risk of gaining neurovirulence while retaining immunogenic properties when used in populations [39, 40], leads to some additional potential vaccine options, with further complexity and trade-offs [35, 41–44]. Recent studies suggest that failures to contain and respond aggressively to type 2 outbreaks are the root cause for OPV2 cessation failure to date [29, 31, 37]. In response to setbacks, in 2018 the GPEI commissioned additional filled doses of Sabin OPV2 [45] and accelerated efforts to develop and produce large supplies of nOPV2 [46]. Some of the mOPV2 bulk procured also went into filled doses of trivalent OPV (tOPV, containing types 1, 2, and 3) for use in Pakistan and Afghanistan [47], which 2018 modeling suggested would likely be needed [48]. Both Pakistan and Afghanistan used tOPV to respond to outbreaks in late 2020 and 2021 [49], and Yemen [50] and Somalia [51] used it in 2022.
The 2022–2026 GPEI strategic plan does not include restarting OPV2 use in RI [52]. The plan assumes successful interruption of global transmission by the end of 2023 of both wild poliovirus type 1 (WPV1) and circulating type 2 VDPVs (cVDPV2s), and briefly discusses ending all use of bivalent OPV (bOPV, containing types 1 and 3) in all RI. The plan states that: “Planning for OPV withdrawal will start at least two years in advance of cessation…[and] address three main issues: strategies for pre-cessation SIAs; the availability of new, more genetically stable vaccine options; and the time-interval between certification of eradication and OPV cessation” [52, page 42]. Modeling that explored the health-economics of the 2022–2026 GPEI plan without bOPV cessation or OPV2 restart as the baseline scenario compared to shifting to control in OPV-using countries using exclusively either IPV or tOPV reported essentially no chance of type 2 transmission dying out [53].
Specific to bOPV cessation, several pre-OPV2 cessation integrated modeling studies discussed differences between the poliovirus types and implications of vaccine choices and OPV cessation timing for supply forecasting [54–56]. An additional study that focused specifically on bOPV cessation highlighted the risks associated with implementation of bOPV cessation and discussed similarities and differences of the 3 poliovirus types [57]. This study also emphasized the need to globally synchronize homotypic OPV cessation, and the importance of ensuring the absence of type 1 and 3 circulating VDPV (cVDPV1 and cVDPV3) transmission prior to cessation, as well as monitoring to confirm complete withdrawal of all doses of the stopped OPV in the supply chain [57]. Most importantly, this study demonstrated that maintaining bOPV preventive (or planned) SIAs (pSIAs) prior to bOPV cessation would substantially lower the risks associated with implementation of bOPV cessation, whereas the use of IPV in RI would only prevent some cases of paralysis [57]. A study published after OPV2 cessation focused on bOPV cessation and characterized the expected increases in vulnerability of populations to importations of types 1 and 3 outbreak viruses and mOPV1 or mOPV3 use for oSIAs as a function of time since bOPV cessation [58], similar to prior studies for OPV2 cessation [24, 25].
Studies of lessons learned from the experience with OPV2 cessation for bOPV cessation highlighted the differences in transmissibility and neurovirulence among poliovirus types [26] and the important role of un- and under-vaccinated subpopulations [59]. Following the global certification of the eradication of indigenous transmission of type 3 wild polioviruses in 2019 [60], which several modeling studies supported by showing high confidence about no circulation given the available surveillance and immunization data [61–64], integrated modeling explored the option of globally coordinating cessation of type 3 OPV (OPV3) prior to type 1 OPV (OPV1) cessation [65]. This study highlighted the importance of coordination with vaccine manufacturers to procure sufficient vaccine supplies while also ensuring the use of vaccines produced, highlighted key differences between types 1 and 3, and suggested potential benefits of coordinating OPV3 cessation prior to OPV1 cessation [65]. However, the 2022–2026 GPEI strategic plan [52] did not consider OPV3 cessation followed by OPV1 cessation as a viable option.
With evidence for ongoing transmission of WPV1 in Pakistan and Afghanistan as of early-2023 and exportations leading to WPV1 cases in Malawi (2021) and Mozambique (2022) [66], the targeted cessation of indigenous WPV1 transmission may not occur by the end of 2023. In addition, even with the potential for faster certification following no detected surveillance evidence of WPV1 transmission as early as 1.5 years after the last reported detection [49, 67, 68], uncertainty remains about when bOPV cessation might occur. However, the World Health Organization (WHO) Strategic Advisory Group of Experts on Immunization (SAGE) recently called for creation of an OPV cessation team [69]. To support the efforts of this team, we applied an integrated model to explore the potential polio endgame trajectory assuming that it includes bOPV cessation in 2027 and it does not include OPV restart. We then discuss bOPV cessation immunization options as of 2022 and explore potential prerequisites (or readiness criteria) that the GPEI may want to consider for bOPV cessation.
2. Methods
We use our updated global poliovirus transmission model to explore the implications of implementing bOPV cessation without coordinated improvements in population immunity, which represents the current global path that we model in this analysis [28, 29, 35, 42–44, 53]. Since the GPEI plan [52] assumes globally-coordinated bOPV cessation by 2027, we assume WPV1 eradication in 2023, followed by global certification of WPV1 eradication, implementation of the planning required for coordinated bOPV cessation, no bOPV intensification, and coordinated bOPV cessation on May 1, 2027. The model divides the world into 72 blocks of 10 subpopulations of approximately 10.7 million total population, stratified by World Bank Income Level (low-income, LI; lower middle-income, LMI; upper middle-income, UMI; high-income, HI) and current vaccine use in RI (i.e., OPV+IPV, IPV/OPV, IPV-only) [28, 29, 35, 42–44, 53]. We further stratify each subpopulation using multiple age groups. Mixing within blocks occurs heterogeneously by age and homogenously in space. Mixing between blocks occurs according to nine varying preferential mixing areas of different size representing larger geographical regions. We consider a prospective analytical time horizon of January 1, 2022 to December 31, 2035, for which we assume current SIA performance characteristics and three vaccine options for post type-specific OPV cessation outbreak responses. Specifically, we assume that oSIAs target children <5 years of age, start 45 days after detection in the model, and include 2 rounds separated by 30 days with 2 additional rounds after breakthrough transmission, with a scope that spans only the outbreak subpopulation when WPV1 R0 <10 or the outbreak subpopulation and its four worst-performing neighbor subpopulations within the same block when WPV1 R0 ≥ 10. In the model, detection occurs at the time of onset of paralysis and without considering additional delays that may exist in practice (e.g., collection of the specimen for laboratory testing, specimen transport and laboratory processing, and/or notification of the case, in the event that that total time required for these takes longer than 45 days) [61]. The oSIA intensity varies for different subpopulations, ranging from 15% true coverage and 95% repeatedly missed probability to 80% true coverage and 50% repeatedly missed probability. We include a scenario: (i) mOPV, which assumes that only type-specific mOPV would be used for outbreak response post type-specific OPV cessation. Considering the current shift toward nOPV2 use for outbreak response and efforts to develop nOPV for types 1 and 3 (i.e., nOPV1 and nOPV3), we consider additional scenarios that assume that nOPV1 and nOPV3 will become available for outbreak response at the time of bOPV cessation. For this analysis, we consider the implications of using nOPV2 starting at the beginning of the time horizon in 2022 for the nOPV scenarios, and that nOPV1 and nOPV3 becomes available at the time of bOPV cessation in 2027, with bOPV used for type 1 and/or 3 oSIAs prior to 2027. However, given uncertainty about nOPV performance in field use, we explore the bounds of the potential trajectories for the novel vaccine. Specifically, we consider the scenarios of (ii) best nOPV, which uses type-specific nOPV for outbreak response assuming the same effectiveness as type-specific mOPV, no reversion despite transmissibility, and no VAPP [35, 41], and (iii) worst nOPV, which uses type-specific nOPV for outbreak response post type-specific OPV cessation, assuming the 90% of the effectiveness of mOPV, prior assumptions for reduced reversion [35], which we further reduced here by 10%, and VAPP occurring at a rate 10% lower than the VAPP rate of mOPV in vaccine recipients. We present the results of the model simulations using the expected value of 100 stochastic iterations, performed using JAVA™ programming language in the integrated development environment Eclipse™, starting with the same random number seeds and initial conditions to control the stochasticity for each scenario to focus on direct comparisons of the different scenarios (i.e., in this analysis, different assumptions about vaccine use of oSIAs).
3. Results
Figure 1(a) shows that following modeled WPV1 eradication in 2023, the total number of expected type 1 paralytic polio cases remains low as long as bOPV use continues. However, with bOPV cessation anticipated in 2027, the model results show a rapid and significant rise in expected type 1 paralytic polio cases. This increase occurs due to: (i) substantially less-than-ideal immunization coverage before modeled globally-coordinated bOPV cessation anticipated in some countries, which provides fertile ground for the highly transmissible and neurovirulent type 1 polioviruses and leads to creation and circulation of cVDPV1, and (ii) expected inadequate outbreak response and no restart of OPV1 use in RI, even in the context of increasing numbers of cases. Thus, this analysis does not include any feedback that might occur if decision makers observe an increase in transmission and they implement different strategies and practices than we assumed here. The use of type 1 mOPV (mOPV1) for oSIAs after bOPV cessation leads to more expected cases than using nOPV1. The introduction of nOPV1 for outbreak response at the time of bOPV cessation comes with a lower risk of seeding new type 1 cVDPVs relative to the risk of seeding using mOPV1, which leads to lower expected cases for both best and worst nOPV1 than with mOPV1. As shown in Figure 1(a), the expected cases after bOPV cessation reflect the insufficient use of bOPV to intensify population immunity prior to bOPV cessation, and the reduction in seeding with even the best nOPV1 does not compensate for the failure to close type 1 immunity gaps prior to bOPV cessation. Overall, these model results suggest that current GPEI plans for OPV1 cessation would likely repeat the experience that occurred with OPV2 cessation. Given discussion of bOPV cessation readiness criteria, if the GPEI insisted on a requirement of ending all cVDPV1 transmission before bOPV cessation, then the model results shown in Figure 1(a) would not apply because they include expected cVDPV1 transmission at the start of the time horizon.
Figure 1.
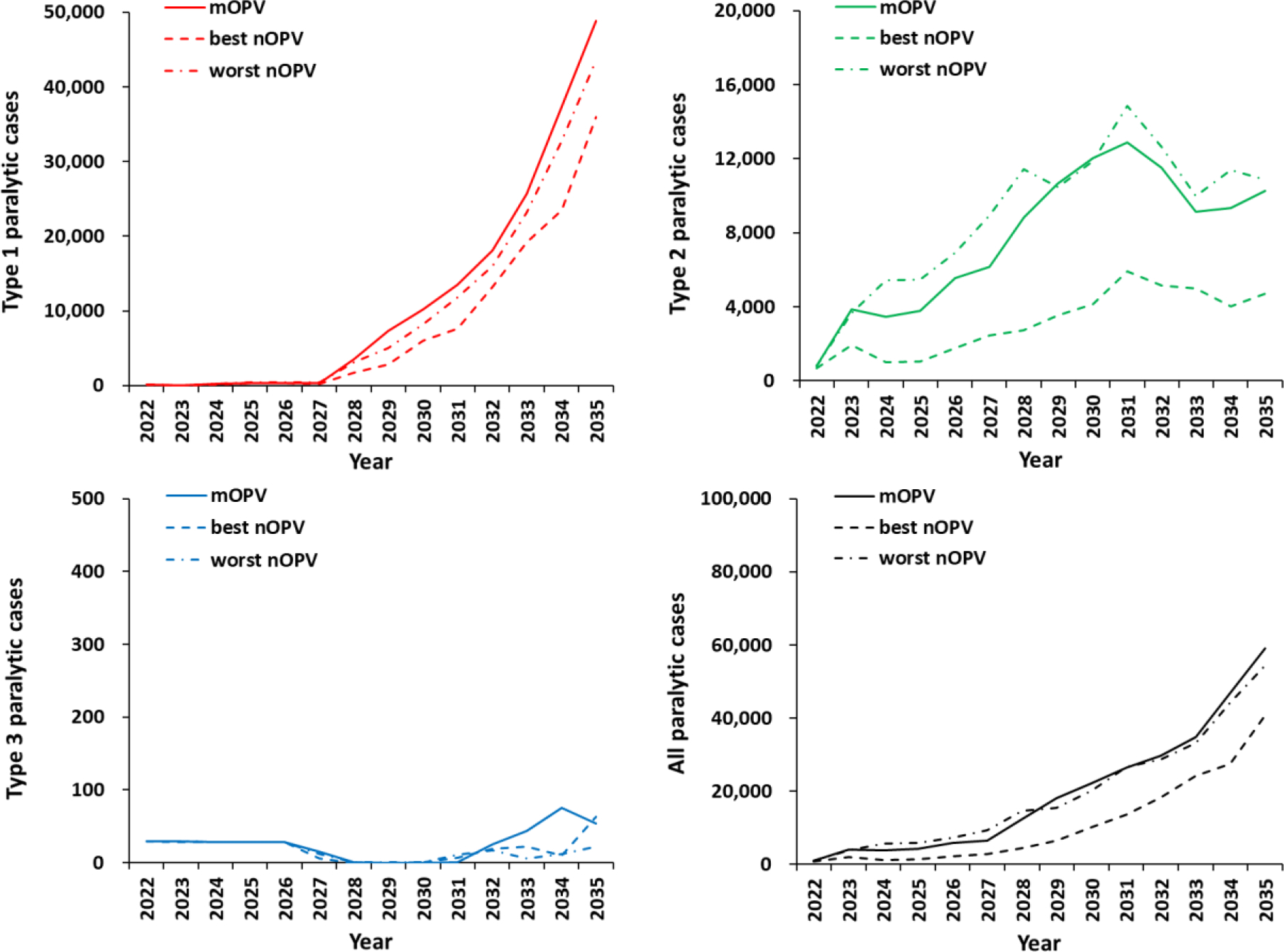
Expected global number of polio cases by year for 100 stochastic iterations of bOPV cessation under the 2022–2026 Strategy (on May 1, 2027) with mOPV (solid line), a best nOPV (dashed line) or a worst nOPV (dotted-dashed line) as the vaccine used for outbreak response.
Given no assumed improvements in outbreak response compared to the current oSIA characteristics, as shown in Figure 1(b), type 2 trajectories remain off track. Replacing mOPV with best nOPV lowers the overall expected burden of paralytic disease, but the model still does not predict poliovirus elimination (i.e., none of the 100 stochastic iterations lead to die out of type 2 transmission at the end of the model time horizon).
Compared to types 1 and 2, early cessation of OPV3 could be considered as a better option as shown in Figure 1(c), although this would depend on addressing logistical, political, economic, and other challenges. Figure 1(d) shows the expected total cases for all 3 poliovirus types. Type 1 poliovirus cases account for most of the global burden, which reflects its relatively greater transmissibility and paralysis-to-infection ratio.
4. OPV cessation immunization options and prerequisites
Prior studies that systematically explored OPV cessation used decision trees to discuss vaccine options [18, 70–73], and characterized the real differences between the choices of national immunization programs for countries of different World Bank income levels. For these discussions and related modeling, the most recent studies classified RI schedules briefly as IPV-only, IPV/OPV sequential, or OPV+IPV, the last of which indicates primary use of OPV but adds a co-administered dose of IPV as indicated by the “+IPV” [28, 71]. For HI and UMI using IPV-only, we assume that they will continue to do so for the foreseeable future [28, 71]. Similarly, for countries currently using an IPV/OPV sequential schedule, we assume that current polio vaccine policies would not change their immunization schedules for the foreseeable future, except that cessation of any of the remaining OPV type(s) would change the formulation of the OPV vaccine they use for the OPV doses, until all OPV cessation occurs and they shift to an IPV-only schedule.
However, for countries that historically relied on OPV only for RI, the current options include increased complexity compared to those in 2019 [28, 71]. Prior to and as a readiness criteria for OPV2 cessation, the SAGE recommended that all countries include at least one dose of IPV in their RI schedules [74], which led to the category of OPV+IPV RI schedules [28, 71]. Building on this prior work, we provide an updated structure of the RI options for the countries using OPV+IPV. We note that some differences would arise in the sets of options if global health leaders decided to either (i) continue [52] or (ii) abandon [5, 6] OPV cessation as the global polio endgame strategy. In both cases, we assume that restarting the use of OPV2 in RI would not occur, and we leave discussion of that topic to other studies [75].
4.1. Continued commitment to globally-coordinated OPV cessation
While prior studies included the theoretical option of OPV1 cessation prior to OPV3 cessation, we exclude it as not under consideration by the GPEI. We implicitly assume that if the world continues to pursue OPV cessation as a strategy, then the efforts will be coordinated [15] and would follow current WHO recommendations. Notably, in October 2020, SAGE recommended a minimum of 2 doses of IPV in all RI schedules [76], and OPV+IPV countries vary with respect to their adoption of this recommendation.
Figure 2 shows the decision tree of the options for countries currently using OPV+IPV RI schedules. We highlight the status quo in bold, and depict the additional permutations of schedules that may arise with the addition of a second dose of IPV by also allowing for the splitting of co-administered doses (i.e., bOPV+IPV) into separate contacts (i.e., bOPV/IPV). Moreover, Figure 2 includes the option that at any point on the path to all OPV cessation, a LMI OPV+IPV countries may prefer to switch to sequential (seq) IPV/OPV schedule instead of including the second IPV dose in its OPV schedule.
Figure 2.
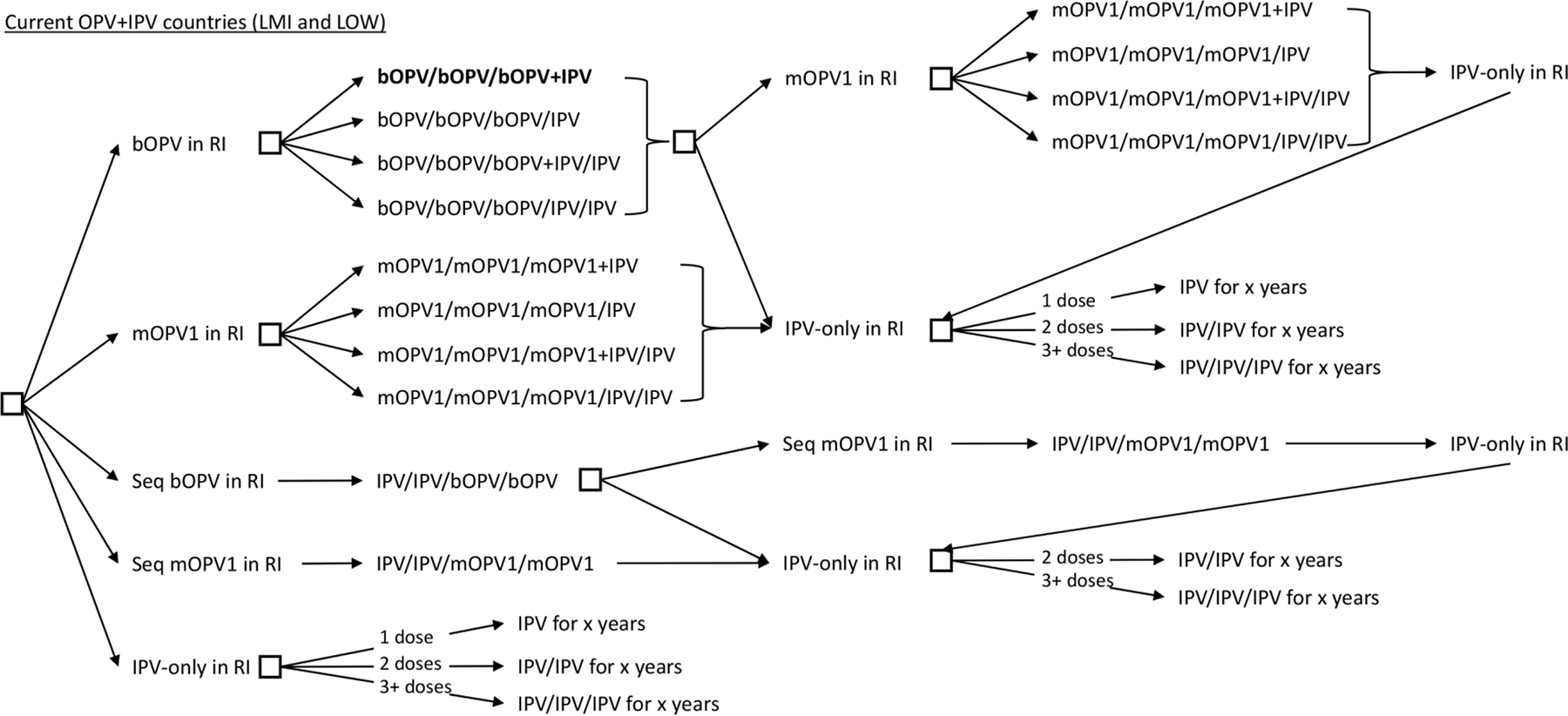
Vaccine options for OPV+IPV in their national immunization programs in 2022: Continued OPV cessation (current status quo shown in bold)
Commitment to OPV cessation includes the decision about when to implement cessation and may depend on prerequisites (i.e., firm requirements) or readiness criteria (i.e., desired conditions). Table 1(a) summarizes some potential options in the left column. In the middle column, we indicate whether the GPEI applied the option in the first column prior to 2016 and the rightmost column indicates whether the readiness criterion was effectively implemented or met prior to 2016 OPV2 cessation [18]. Global certification of the eradication of indigenous transmission of WPV1 represents a prerequisite, as already occurred in 2015 for type 2 [77] and 2019 for type 3 [60]. Requiring the end of transmission of cVDPV transmission for types 1 and/or 3 prior to homotypic OPV cessation would help to reduce the risks of the coordinated OPV cessation failing, although questions remain about whether, when, and how to verify or validate the end of cVDPV transmission [78]. Prerequisites or readiness criteria for bOPV cessation may include increasing population immunity to transmission prior to OPV cessation by performing homotypic OPV pSIAs intensively between now and/or in the months running up to OPV cessation, and/or achieving immunization coverage targets for OPV and/or IPV in RI. The development of a vaccine stockpile of homotypic mOPV and/or nOPV for outbreak response prior to OPV cessation could represent another anticipated prerequisite, as well as instituting specific requirements for surveillance.
Table 1.
Options for prerequisites or readiness criteria for OPV cessation
| (a) Prerequisite or readiness criteria prior to 2016 OPV2 cessation and completion status prior to OPV2 cessation | ||
|---|---|---|
| Option | Prior | Done |
| Global certification of the eradication of indigenous homotypic WPV | Y | Y |
| Stockpile(s) homotypic OPV (Sabin) vaccine for outbreak response | Y | Y |
| End transmission of homotypic cVDPV prior to homotypic cessation | Y | N |
| Increase homotypic population immunity to transmission prior to cessation | Y | N |
| Develop protocols and needed financial resources for outbreak response | Y | N |
| Achieve specific minimum immunization dose or coverage targets using IPV | Y | N |
| Achieve specific minimum immunization dose or coverage targets using OPV | N | |
| Stockpile(s) homotypic nOPV vaccine for outbreak response | N | |
| Institute requirements for surveillance | N | |
| Implement containment for homotypic WPV | N | |
| (b) Risk management strategies that could represent post-OPV cessation prerequisites or readiness criteria | ||
| Develop plans for OPV restart that should include specific trigger(s) | ||
| Ensure accountable management of outbreak response resources | ||
| Ensure sufficient quantities of polio vaccines needed for national RI programs | ||
| Implement plans to manage risks of immunodeficiency-associated VDPV | ||
| Develop communication strategies to facilitate risk management | ||
| Stockpile(s) homotypic novel OPV vaccine for outbreak response | ||
Table 1(b) includes potential post-cessation risk management strategies. The experience with OPV2 cessation should motivate revisions and/or the development of explicit and detailed plans for risk management to address the previously encountered problems that now represent known risks. These strategies should recognize the potential for cessation and outbreak response failure and identify the potential triggers for OPV restart, if needed. Additional lessons from OPV2 cessation to date may also include efforts to ensure accountable management of outbreak response resources (e.g., vaccine doses in stockpiles and logistical, technical, and/or financial support) and/or the need to ensure access to sufficient quantities of polio vaccines needed for national RI programs as vaccine schedules evolve after cessation. The development of communication strategies related to risk management, including the possibility of OPV restart, should also become part of the post-cessation strategy. Long-term risk management also will require the development and implementation of plans to manage risks posed by immunodeficiency-associated VDPV excreters and sustaining surveillance to rapidly detect any future poliovirus events.
The adoption of prerequisites could possibly delay the implementation of OPV cessation if adequate time for preparation is not provided, which would substantially increase the costs for polio immunization and surveillance in the run-up to OPV cessation.
4.2. Abandoned globally-coordinated OPV cessation
As an alternative, even if unlikely, in the event that the currently unsuccessful experience with OPV2 cessation leads to the decision not to pursue globally-coordinated OPV cessation as a polio endgame strategy, then country leaders could choose the path of coordinated or uncoordinated control. Figure 3 shows the decision tree for these options, while still assuming no OPV2 restart in RI. The tree includes the possibility of using bOPV-only in RI, which would imply type 2-containing poliovirus vaccine use would only occur in the event of an outbreak. Coordinated control would assume that the GPEI, or some successor group, would continue to manage the globally interdependent risks associated with poliovirus transmission, and that this group would maintain some capacity related to outbreak response and polio vaccine stockpile management for type 2 outbreak response, since we have not assumed that OPV2 use would restart in RI. Notably, in the absence of a continued global commitment to eradication, coordinated control and continuance of current GPEI activities and sufficient resources to support them appears unlikely, which would imply uncoordinated control.
Figure 3.
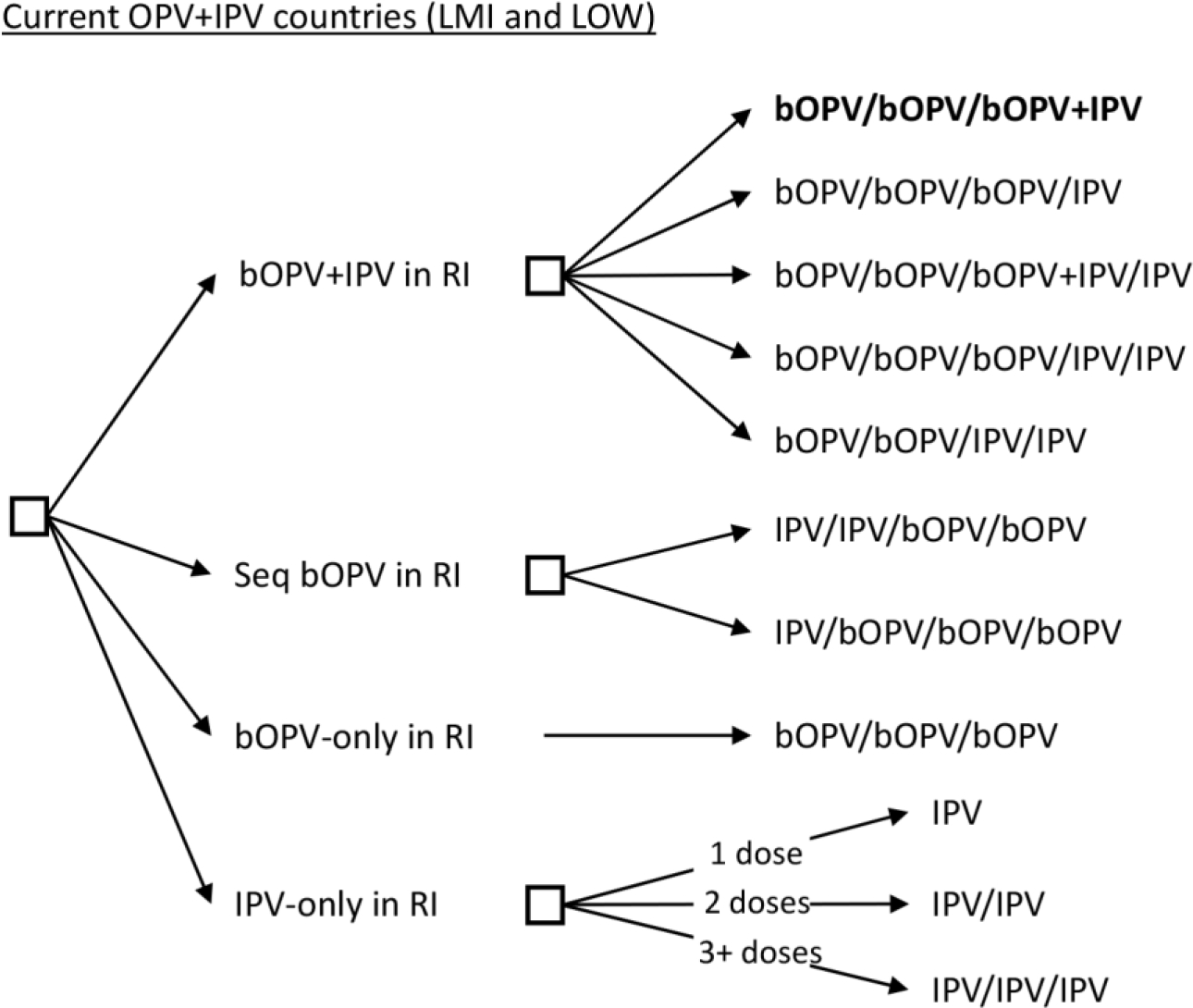
Vaccine options for OPV+IPV in their national immunization programs in 2022: Abandoned OPV cessation without OPV2 restart in RI (current status quo shown in bold)
National leaders could maintain continued use of both OPV and IPV, either with the current OPV-first approach (i.e., OPV+IPV) or by shifting to a sequential approach (i.e., IPV/bOPV) to mitigate the risks of VAPP. Similar to the options shown in Figure 2, we assume that countries could decide to add a second IPV dose, as SAGE has recommended, and that bOPV and IPV co-administration may or may not occur. Alternatively, they could opt just to use bOPV only and stop their use of IPV. Finally, national leaders could decide to shift to using only IPV, for which they would need to achieve very high coverage to keep population immunity high to avert the risks of large outbreaks after poliovirus importation or VDPV emergence. Achieving and maintaining high population immunity would imply high costs of control. Even with a global decision to abandon OPV cessation as a polio endgame strategy, individual countries or regions may still choose to coordinate OPV cessation within their borders. However, the risks of importation of OPV-related viruses from areas that do not stop OPV use would pose an on-going threat [15].
Even in countries with very high IPV-only coverage, imported live polioviruses may sustain some transmission in populations and potentially cause paralytic cases in under-vaccinated subpopulations [79–82]. Recent detections of polio transmission in the UK [83], Israel [84] and the US [85, 86] in un- or under-immunized populations provide proof of concept. Thus, in the event that OPV use continues, countries will need to consider their national immunization policies and decide which vaccines and how many doses to deliver as part of RI. For middle- income countries using multiple doses of bOPV plus one or two doses of IPV in RI, the decision to revert to only using three doses of a bOPV formulation may prove more attractive from a financial perspective than continuing to deliver multiple OPV and IPV doses [34]. Alternatively, with the development of hexavalent vaccines that contain IPV, countries may prefer to rely on only IPV in this type of combined vaccine for RI, and to use OPV only if and when needed for outbreak response, if OPV supplies remain available in the absence of any regular demand for RI.
4.3. Implications for forecasting polio vaccine needs
The substantial differences in the current options with respect to potential RI vaccine needs, even without OPV2 restart in RI, make efforts to forecast vaccine demand very challenging. This may create issues related to over- or under-supply of specific polio vaccines due to small numbers of manufacturers who face unclear market incentives. National policies developed by large self-producing countries, like China, India, and the Russian Federation, will likely affect the large populations in these countries as well as influence vaccine options available to other populations that might import their poliovirus vaccines.
5. Discussion
The current status of type 2 poliovirus transmission, with nearly 3,000 reported cVDPV2 cases since 2016 (as of April 3, 2023) [38] continues to lead to questions about OPV cessation as a global polio endgame strategy as well as the role of nOPV. The accelerated development of nOPV2 for outbreak response, now in use under an Emergency Use Listing (EUL) [46], is being followed by a similarly accelerated development of genetically modified nOPV strains for types 1 and 3 (i.e., nOPV1 and nOPV3), and consideration of a potential trivalent nOPV product. Given the absence of performance data for these products in development, we leave discussion of those options to future studies.
Our assumption in this analysis of no restart of OPV2 (i.e., either Sabin OPV2 or nOPV2) in RI represents the current GPEI strategy. We note that in the event of OPV2 restart in RI, the possibility of multivalent OPV formulations could quickly become even more challenging for national immunization program managers and vaccine manufacturers to navigate due to the large number of possible OPV-containing vaccines. The combinatoric multivalent combinations after OPV2 restart could include simply returning to Sabin-strain tOPV with its predictable properties, using only nOPVs (e.g., nOPV1, nOPV2, and/or nOPV3 independently or in various possible combinations), or using both Sabin OPVs and nOPVs (e.g., a trivalent OPV containing novel strains of all types of OPVs or Sabin bOPV and nOPV2). We do not provide the exhaustive list of combinations of potential OPV options here, but highlight the anticipated complexity discussed elsewhere [75]. If OPV2 restart occurs, then countries might change their willingness to pay for multiple doses of IPV, particularly if a trivalent OPV formulation again becomes available. Prior to the development of combination OPV products containing any nOPV, clinical trials could be required to demonstrate the non-inferiority of the combination schedule compared to the schedule with individual OPV components to support licensure. This could theoretically become very complicated given the number of possible combinations. In practice, the possible set narrows considerably due to unlikely licensure of mOPV1 for RI in the foreseeable future (required for OPV3 cessation if it occurred), and unlikely development of novel bivalent products. In addition, the current lack of full licensure of nOPV2 (with licensure anticipated for use in outbreak response as early as the first quarter of 2023 [46], but not yet obtained) and restrictions on its use by the GPEI under the EUL that preclude co-administration with other OPVs [87], may make studies related to co-administration more challenging. Thus, any RI schedules that include OPV2 (mOPV2 or nOPV2) would need to occur after a global decision to restart OPV2 use in RI.
Although we mentioned the potential prerequisite of requiring the end of transmission of type 1 and 3 cVDPVs prior to homotypic OPV cessation as one potential post-cessation risk reduction strategy, confidence about no circulation of these will only come as the time since cessation increases and with no observed signals of transmission despite ongoing and well-performing surveillance [61, 68, 78]. Challenges to achieving high confidence about meeting such a requirement and the OPV2 cessation experience may motivate discussions about introducing novel OPV strains for types 1 and 3 prior to bOPV cessation.
The importance of effective containment of all live polioviruses also emerges after OPV cessation. The poor outcomes observed to date with respect to managing type 2 live poliovirus risks after OPV2 cessation [27–31] should lead to questions about effective removal of all OPV from supply chains and the field, containment, and stockpile management. In the context of any potential ongoing nOPV use, such that children in some areas may inadvertently continue to receive oral doses of poliovirus vaccines, containment issues may continue to face challenges related to the delivery of different formulations.
The risks of bOPV cessation based on the current polio endgame trajectory (Figure 1), include substantial type 1 poliovirus transmission, including type 1 cVDPVs, with potentially large consequences given the high transmissibility of these viruses. The introduction of IPV prior to OPV2 cessation did not reduce the consequences of OPV2 cessation substantially, in part due to low IPV coverage achieved and delays in introduction as well as limited IPV effect on intestinal immunity. Efforts to develop combination vaccines that contain IPV for use in RI for low- and middle-income countries may offer the potential to achieve higher coverage with IPV-containing vaccines, but this will come with added costs [34, 88].
As discussed in 2018 [27], the ramp down of the global polio budget led to diminished capacities and performance of both preventive and outbreak immunization activities, since countries did not self-fund continued activities [89]. The reduction in polio eradication-dedicated staff [89], disruptions in polio immunization and surveillance activities during COVID-19, and the failure of the 2016 switch from tOPV to bOPV to end cVDPV2 outbreaks to date create substantial hurdles. Although the GPEI partners remain committed to OPV cessation as the global strategy, the failure to complete successful OPV2 cessation to date and the results of this analysis may affect perceptions about the potential for successful bOPV cessation and motivate demands for a different bOPV cessation or polio endgame strategy.
Related to OPV2 cessation, in 2017 we expected that within a few years, population immunity to type 2 transmission in OPV using countries would continue to decrease, and that the fraction of the population with no type 2 vaccine protection would accumulate to a level that re-established endemic transmission of cVDPV2s could occur [90]. Our observations of poor performance with respect to the operational management of many outbreak responses following OPV2 cessation as of early 2019 led to discussions about the potential need to plan for restarting the OPV2 vaccine production and use in RI [71]. In 2019, prior modeling did not anticipate successful OPV2 cessation given GPEI performance and plans at the time [71]. Notably, in response to the epidemiological situation and programmatic challenges, in 2018, the GPEI partners procured additional bulk of mOPV2 [45], and in 2019 they accelerated efforts to develop and produce nOPV2 [46]. However, the GPEI partners did not abandon OPV cessation as a strategy and have not recommended restarting OPV2 use in RI, to date. If nothing changes in the operational management of outbreak responses, the model shows essentially no chance of successful poliomyelitis eradication, which requires cessation of all OPV use (including potential repeated cessation of OPV2, both Sabin and novel). With continued suggestions by some to limit the global polio eradication goal to certification of eradication of polio cases caused by the transmission of all three wild polioviruses (disease) and allowing continued use of OPV [5, 6], we highlight the inability to achieve the eradication of all polio cases using nOPV given the observations of paralytic cases (both VAPP and cVDPV) associated with nOPV2 use. With the challenges to date and that continue to arise, questions may arise about the feasibility of implementing strategies and tactics that can successfully implement OPV cessation. The increasing use of nOPV2 (and potential future use of nOPV1 and/or nOPV3) may create some new opportunities, but may also lead to substantial challenges related to managing vaccine supplies and expectations related to cases and costs.
Urgently stopping all cVDPV2 transmission by implementing necessary operational changes represents a priority of the GPEI strategic plan [52]. Regarding prospective OPV withdrawal options, GPEI partners and stakeholders need to review the possibilities and make transparent, carefully considered, and globally-coordinated decisions, vaccine policies, and risk management choices. This analysis highlights the importance of risk management activities and contingency planning. The currently decentralized GPEI structure and emphasis on 12-month budgets and planning cycles presents challenges for longer-term forecasting by modelers, analysts, national and international policy makers, vaccine manufacturers, and others. Without a clear path and contingent alternatives declared in the near future, and given the continued expansion of the available vaccine options (e.g., nOPVs, IPV combination formulations), the polio eradication endgame will become increasingly uncertain and difficult to manage and model.
6. Conclusion
As the polio endgame increases in complexity and continued delays extend the timelines for ending all poliovirus transmission, the potential consequences do not currently support global coordination of bOPV cessation in 2027 due to the need to substantially increasing population immunity using bOPV in many countries prior to implementation of the endgame strategy.
Funding
The first and last two authors acknowledge support for this publication under Cooperative Agreement Number NU2RGH001915-02-00 funded by the Centers for Disease Control and Prevention. The views expressed are solely those of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or Department of Health and Human Services.
References
- 1.World Health Organization. Global eradication of poliomyelitis: Report of the second meeting of the Global Technical Consultative Group (TCG), 28 April 1997. 1997 [cited 2023 February 24, 2003]; Available from: https://apps.who.int/iris/bitstream/handle/10665/63994/WHO_EPI_GEN_98.04.pdf. [Google Scholar]
- 2.Duintjer Tebbens RJ, et al. , Risks of paralytic disease due to wild or vaccine-derived poliovirus after eradication. Risk Anal, 2006. 26(6): p. 1471–505. [DOI] [PubMed] [Google Scholar]
- 3.John TJ, Two good reasons to drop type 2 virus from oral polio vaccine. Lancet, 2004. 364(9446): p. 1666. [DOI] [PubMed] [Google Scholar]
- 4.John TJ and Dharmapalan D, Challenges en route to polio eradication. Lancet, 2022. 400(10350): p. 428–429. [DOI] [PubMed] [Google Scholar]
- 5.Chumakov K, et al. , Vaccination against polio should not be stopped. Nat Rev Microbiol, 2007. 5(12): p. 952–8. [DOI] [PubMed] [Google Scholar]
- 6.Chumakov K, et al. , Polio eradication at the crossroads. Lancet Glob Health, 2021. 9(8): p. e1172–e1175. [DOI] [PubMed] [Google Scholar]
- 7.Joffe AM, et al. , NIAID workshop on secondary vaccine effects. Nat Immunol, 2021. 22(11): p. 1363–1366. [DOI] [PubMed] [Google Scholar]
- 8.Chumakov K, et al. , Can existing live vaccines prevent COVID-19? Science, 2020. 368(6496): p. 1187–1188. [DOI] [PubMed] [Google Scholar]
- 9.Aaby P, et al. , Oral polio vaccination and low case fatality at the paediatric ward in Bissau, Guinea-Bissau. Vaccine, 2004. 22(23–24): p. 3014–7. [DOI] [PubMed] [Google Scholar]
- 10.Thompson KM and Badizadegan K, Health economic analyses of secondary vaccine effects: a systematic review and policy insights. Expert Rev Vaccines, 2022. 21(3): p. 297–312. [DOI] [PubMed] [Google Scholar]
- 11.Thompson KM, Kalkowska DA, and Badizadegan K, A Health Economic Analysis for Oral Poliovirus Vaccine to Prevent COVID-19 in the United States. Risk Anal, 2021. 41(2): p. 376–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson KM, Kalkowska DA, and Badizadegan K, No Role for Reintroducing OPV into the United States with Respect to Controlling COVID-19 [Response to the letter to the Editor by Chumakov et al.]. Risk Anal, 2021. 41(2): p. 389–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson KM and Kalkowska DA, Review of poliovirus modeling performed from 2000 to 2019 to support global polio eradication. Expert Rev Vaccines, 2020. 19(7): p. 661–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson KM, et al. , The risks, costs, and benefits of possible future global policies for managing polioviruses. Am J Public Health, 2008. 98(7): p. 1322–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson KM and Duintjer Tebbens RJ, The case for cooperation in managing and maintaining the end of poliomyelitis: stockpile needs and coordinated OPV cessation. Medscape J Med, 2008. 10(8): p. 190. [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson KM, Duintjer Tebbens RJ, and Pallansch MA, Evaluation of response scenarios to potential polio outbreaks using mathematical models. Risk Anal, 2006. 26(6): p. 1541–56. [DOI] [PubMed] [Google Scholar]
- 17.World Health Assembly. Poliomyelitis: mechanism for management of potential risks to eradication (resolution 61.1), WHO, Geneva, 19–24 May 2008. 2008 February 24, 2023]; Available from: http://apps.who.int/gb/ebwha/pdf_files/WHA61-REC1/A61_Rec1-part2-en.pdf. [Google Scholar]
- 18.Thompson KM and Tebbens RJ, Current polio global eradication and control policy options: perspectives from modeling and prerequisites for oral poliovirus vaccine cessation. Expert Rev Vaccines, 2012. 11(4): p. 449–59. [DOI] [PubMed] [Google Scholar]
- 19.Duintjer Tebbens RJ, et al. , An economic analysis of poliovirus risk management policy options for 2013–2052. BMC Infect Dis, 2015. 15: p. 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duintjer Tebbens RJ, et al. , Characterization of outbreak response strategies and potential vaccine stockpile needs for the polio endgame. BMC Infect Dis, 2016. 16: p. 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson KM and Duintjer Tebbens RJ, Modeling the dynamics of oral poliovirus vaccine cessation. J Infect Dis, 2014. 210 Suppl 1: p. S475–84. [DOI] [PubMed] [Google Scholar]
- 22.Duintjer Tebbens RJ and Thompson KM, Modeling the potential role of inactivated poliovirus vaccine to manage the risks of oral poliovirus vaccine cessation. J Infect Dis, 2014. 210 Suppl 1: p. S485–97. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization Global Polio Eradication Initiative. Standard operating procedures: Responding to a poliovirus event or outbreak: Part 1: General SOPs. 2016. February 24, 2023]; Available from: https://s3.amazonaws.com/gpei-tk/reference_links/en/GPEI_SOPs.pdf. [Google Scholar]
- 24.Duintjer Tebbens RJ, Hampton LM, and Thompson KM, Implementation of coordinated global serotype 2 oral poliovirus vaccine cessation: risks of inadvertent trivalent oral poliovirus vaccine use. BMC Infect Dis, 2016. 16: p. 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duintjer Tebbens RJ, Hampton LM, and Thompson KM, Implementation of coordinated global serotype 2 oral poliovirus vaccine cessation: risks of potential non-synchronous cessation. BMC Infect Dis, 2016. 16: p. 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson KM and Duintjer Tebbens RJ, Lessons From Globally Coordinated Cessation of Serotype 2 Oral Poliovirus Vaccine for the Remaining Serotypes. J Infect Dis, 2017. 216(suppl_1): p. S168–S175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duintjer Tebbens RJ and Thompson KM, Polio endgame risks and the possibility of restarting the use of oral poliovirus vaccine. Expert Rev Vaccines, 2018. 17(8): p. 739–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalkowska DA, et al. , Global Transmission of Live Polioviruses: Updated Dynamic Modeling of the Polio Endgame. Risk Anal, 2021. 41(2): p. 248–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalkowska DA, et al. , Updated Characterization of Post-OPV Cessation Risks: Lessons from 2019 Serotype 2 Outbreaks and Implications for the Probability of OPV Restart. Risk Anal, 2021. 41(2): p. 320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson KM, Polio eradication: what kind of world do we want? Lancet Infect Dis, 2022. 22(2): p. 161–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macklin GR, et al. , Evolving epidemiology of poliovirus serotype 2 following withdrawal of the serotype 2 oral poliovirus vaccine. Science, 2020. 368(6489): p. 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson KM and Kalkowska DA, Reflections on Modeling Poliovirus Transmission and the Polio Eradication Endgame. Risk Anal, 2021. 41(2): p. 229–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization Global Polio Eradication Initiative. Polio eradication and endgame Strategic Plan (2013–2018). Geneva; 2013. Report No: WHO/POLIO/13.02 2013 [cited 2019 Jun 4]; Available from: http://polioeradication.org/wp-content/uploads/2016/07/PEESP_EN_A4.pdf [Accesssed February 24, 2023]. [Google Scholar]
- 34.Kalkowska DA and Thompson KM, Health and Economic Outcomes Associated with Polio Vaccine Policy Options: 2019–2029. Risk Anal, 2021. 41(2): p. 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalkowska DA, et al. , Updated Characterization of Outbreak Response Strategies for 2019–2029: Impacts of Using a Novel Type 2 Oral Poliovirus Vaccine Strain. Risk Anal, 2021. 41(2): p. 329–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson KM, Modeling and Managing Poliovirus Risks: We are Where we are. Risk Anal, 2021. 41(2): p. 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson KM, Kalkowsa DA, and Badizadegan K, ooking back at prospective modeling of outbreak response strategies for managing global type 2 oral poliovirus vaccine (OPV2) cessation Front Public Health, 2023. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization Global Polio Eradication Initiative. Circulating vaccine-derived poliovirus. 2023. April 3, 2023]; Available from: https://polioeradication.org/wp-content/uploads/2023/03/weekly-polio-analyses-cVDPV-20230328.pdf. [Google Scholar]
- 39.Konopka-Anstadt JL, et al. , Development of a new oral poliovirus vaccine for the eradication end game using codon deoptimization. NPJ Vaccines, 2020. 5(1): p. 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeh MT, et al. , Engineering the Live-Attenuated Polio Vaccine to Prevent Reversion to Virulence. Cell Host Microbe, 2020. 27(5): p. 736–751 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson KM, Kalkowska DA, and Badizadegan K, Hypothetical emergence of poliovirus in 2020: part 2. exploration of the potential role of vaccines in control and eradication. Expert Rev Vaccines, 2021. 20(4): p. 449–460. [DOI] [PubMed] [Google Scholar]
- 42.Kalkowska DA, et al. , The impact of disruptions caused by the COVID-19 pandemic on global polio eradication. Vaccine, 2023. 41 Suppl 1: p. A12–A18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalkowska DA, et al. , Serotype 2 oral poliovirus vaccine (OPV2) choices and the consequences of delaying outbreak response. Vaccine, 2023. 41 Suppl 1: p. A136–A141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalkowska DA, et al. , Outbreak response strategies with type 2-containing oral poliovirus vaccines. Vaccine, 2023. 41 Suppl 1: p. A142–A152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Health Organization and UNICEF. 17th WHO/UNICEF Consultation with OPV/IPV Manufacturers and National Authorities for Containment of Polio Vaccine Producing Countries. 2018. February 21, 2019]; Available from: http://polioeradication.org/wp-content/uploads/2018/11/2018_WHO_UNICEF_Consultation_Mtg_Report_FINAL.pdf. [Google Scholar]
- 46.Macklin GR, et al. , Enabling accelerated vaccine roll-out for Public Health Emergencies of International Concern (PHEICs): Novel Oral Polio Vaccine type 2 (nOPV2) experience. Vaccine, 2023. 41 Suppl 1: p. A122–A127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Global Polio Eradication Initiative. GPEI consultation with OPV/IPV manufacturers, national authorities for containment and national regulatory authorities. 2020. September 29, 2022]; Available from: https://polioeradication.org/wp-content/uploads/2022/01/2020-GPEI-Consultation-with-OPV-IPV-Manufacturers-NACs-NRAs.pdf. [Google Scholar]
- 48.Duintjer Tebbens RJ, et al. , Modeling Poliovirus Transmission in Pakistan and Afghanistan to Inform Vaccination Strategies in Undervaccinated Subpopulations. Risk Anal, 2018. 38(8): p. 1701–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalkowska DA, Badizadegan K, and Thompson KM, Modeling scenarios for ending poliovirus transmission in Pakistan and Afghanistan. Risk Anal, 2022: p. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Web Relief. UNICEF Yemen humanitarian situation report: 1 – 31 March 2022. 2022. June 7, 2022]; Available from: https://reliefweb.int/report/yemen/unicef-yemen-humanitarian-situation-report-1-31-march-2022. [Google Scholar]
- 51.Global Polio Eradication Initiative. Somalia. 2022. October 4, 2022]; Available from: https://polioeradication.org/where-we-work/somalia/. [Google Scholar]
- 52.World Health Organization Global Polio Eradication Initiative. Polio eradication strategy 2022–2026: Delivering on a promise. 2021. Feb 24, 2022]; Available from: https://polioeradication.org/wp-content/uploads/2022/06/Polio-Eradication-Strategy-2022-2026-Delivering-on-a-Promise.pdf. [Google Scholar]
- 53.Thompson KM, Kalkowska DA, and Badizadegan K, Health economic analysis of vaccine options for the polio eradication endgame: 2022–2036. Expert Rev Vaccines, 2022. 21(11): p. 1667–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duintjer Tebbens RJ and Thompson KM, Managing the risk of circulating vaccine-derived poliovirus during the endgame: oral poliovirus vaccine needs. BMC Infect Dis, 2015. 15: p. 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson KM and Duintjer Tebbens RJ, The differential impact of oral poliovirus vaccine formulation choices on serotype-specific population immunity to poliovirus transmission. BMC Infect Dis, 2015. 15: p. 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thompson KM and Duintjer Tebbens RJ, Health and economic consequences of different options for timing the coordinated global cessation of the three oral poliovirus vaccine serotypes. BMC Infect Dis, 2015. 15: p. 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tebbens RJD, et al. , Maintenance and Intensification of Bivalent Oral Poliovirus Vaccine Use Prior to its Coordinated Global Cessation. J Vaccines Vaccin, 2016. 7(5): p. 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duintjer Tebbens RJ, Hampton LM, and Thompson KM, Planning for globally coordinated cessation of bivalent oral poliovirus vaccine: risks of non-synchronous cessation and unauthorized oral poliovirus vaccine use. BMC Infect Dis, 2018. 18(1): p. 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thompson KM and Duintjer Tebbens RJ, Lessons From the Polio Endgame: Overcoming the Failure to Vaccinate and the Role of Subpopulations in Maintaining Transmission. J Infect Dis, 2017. 216(suppl_1): p. S176–S182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.World Health Organization. Report from the Twentieth Meeting of the Global Commission for Certification of Poliomyelitis Eradication, Geneva, Switzerland, 17–18 October 2019. 2019 Dec 31, 2019]; Available from: http://polioeradication.org/wp-content/uploads/2016/07/20th-meeting-of-the-Global-Commission-for-the-Certification-of-Eradication-of-Poliomyelitis-17-18-October-2019.pdf. [Google Scholar]
- 61.Kalkowska DA, et al. , Modeling undetected live poliovirus circulation after apparent interruption of transmission: implications for surveillance and vaccination. BMC Infect Dis, 2015. 15: p. 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kalkowska DA, et al. , Modeling Undetected Live Poliovirus Circulation After Apparent Interruption of Transmission: Pakistan and Afghanistan. Risk Anal, 2019. 39(2): p. 402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kalkowska DA, Duintjer Tebbens RJ, and Thompson KM, Environmental Surveillance System Characteristics and Impacts on Confidence About No Undetected Serotype 1 Wild Poliovirus Circulation. Risk Anal, 2019. 39(2): p. 414–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duintjer Tebbens RJ, Kalkowska DA, and Thompson KM, Global certification of wild poliovirus eradication: insights from modelling hard-to-reach subpopulations and confidence about the absence of transmission. BMJ Open, 2019. 9(1): p. e023938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kalkowska DA and Thompson KM, Expected Implications of Globally Coordinated Cessation of Serotype 3 Oral Poliovirus Vaccine (OPV) Before Serotype 1 OPV. Risk Anal, 2021. 41(2): p. 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.World Health Organization. Wild poliovirus list: List of wild poliovirus by country and year. 2023. April 3, 2023]; {Kalkowsa, 2023 #578}:[Available from: https://polioeradication.org/polio-today/polio-now/wild-poliovirus-list/. [Google Scholar]
- 67.Kalkowska DA, et al. , Updated Characterization of Poliovirus Transmission in Pakistan and Afghanistan and the Impacts of Different Outbreak Response Vaccine Options. J Infect Dis, 2021. 224(9): p. 1529–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kalkowska DA, Badizadegan K, and Thompson KM, Modeling undetected live type 1 wild poliovirus circulation after apparent interruption of transmission: Pakistan and Afghanistan. Risk Anal, 2022: p. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.World Health Organization, Meeting of the Strategic Advisory Group of Experts on immunization, April 2022: conclusions and recommendations. Weekly Epidemiological Record, 2022. 97(24): p. 274–275. [Google Scholar]
- 70.Sangrujee N, et al. , Policy decision options during the first 5 years following certification of polio eradication. MedGenMed, 2003. 5(4): p. 35. [PubMed] [Google Scholar]
- 71.Thompson KM and Kalkowska DA, Logistical challenges and assumptions for modeling the failure of global cessation of oral poliovirus vaccine (OPV). Expert Rev Vaccines, 2019. 18(7): p. 725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thompson KM, et al. , Preeradication vaccine policy options for poliovirus infection and disease control. Risk Anal, 2013. 33(4): p. 516–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thompson KM and Duintjer Tebbens RJ, National choices related to inactivated poliovirus vaccine, innovation and the endgame of global polio eradication. Expert Rev Vaccines, 2014. 13(2): p. 221–34. [DOI] [PubMed] [Google Scholar]
- 74.World Health Organization, Meeting of the Strategic Advisory Group of Experts on immunization, October 2014 - conclusions and recommendations. Weekly Epidemiological Record, 2014. 89(50): p. 561–576. [PubMed] [Google Scholar]
- 75.Kalkowsa DA, et al. , Complexity of options related to restarting oral poliovirus vaccine (OPV) in national immunization programs after OPV cessation. Gates Open Res, 2023: p. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.World Health Organization, Meeting of the Strategic Advisory Group of Experts on immunization, October 2015 - conclusions and recommendations. Wkly Epidemiol Rec, 2015. 90(50): p. 681–99. [PubMed] [Google Scholar]
- 77.World Health Organization. 14th Meeting of the Global Commission for Certification of Poliomyelitis Eradication (GCC), Bali, Indonesia, 20–21 September, 2015. Summary of findings, decisions and recommendations. 2015 Jul 22, 2021]; Available from: http://polioeradication.org/wp-content/uploads/2016/07/1Report.pdf. [Google Scholar]
- 78.World Health Organization. Report from the Twenty-second Meeting of the Global Commission for Certification of Poliomyelitis Eradication, Geneva, Switzerland, 3 June 2022. 2022 September 12, 2022]; Available from: https://polioeradication.org/wp-content/uploads/2022/09/22nd-GCC-report-20220907.pdf. [Google Scholar]
- 79.Duintjer Tebbens RJ, et al. , A dynamic model of poliomyelitis outbreaks: learning from the past to help inform the future. Am J Epidemiol, 2005. 162(4): p. 358–72. [DOI] [PubMed] [Google Scholar]
- 80.Thompson KM, et al. , Trends in the risk of U.S. polio outbreaks and poliovirus vaccine availability for response. Public Health Rep, 2012. 127(1): p. 23–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kalkowska DA, et al. , Modeling options to manage type 1 wild poliovirus imported into Israel in 2013. J Infect Dis, 2015. 211(11): p. 1800–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thompson KM, Kalkowska DA, and Duintjer Tebbens RJ, Managing population immunity to reduce or eliminate the risks of circulation following the importation of polioviruses. Vaccine, 2015. 33(13): p. 1568–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.United Kingdom Department of Health & Social Care. Joint Committee on Vaccination and Immunisation statement on vaccination strategy for the ongoing polio incident. 2022. Aug 24, 2022]; Available from: https://www.gov.uk/government/publications/vaccination-strategy-for-ongoing-polio-incident-jcvi-statement/joint-committee-on-vaccination-and-immunisation-statement-on-vaccination-strategy-for-the-ongoing-polio-incident. [Google Scholar]
- 84.Zuckerman NS, et al. , Emergence of genetically linked vaccine-originated poliovirus type 2 in the absence of oral polio vaccine, Jerusalem, April to July 2022. Euro Surveill, 2022. 27(37). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Link-Gelles R, et al. , Public Health Response to a Case of Paralytic Poliomyelitis in an Unvaccinated Person and Detection of Poliovirus in Wastewater - New York, June-August 2022. MMWR Morb Mortal Wkly Rep, 2022. 71(33): p. 1065–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ryerson AB, et al. , Wastewater Testing and Detection of Poliovirus Type 2 Genetically Linked to Virus Isolated from a Paralytic Polio Case - New York, March 9-October 11, 2022. MMWR Morb Mortal Wkly Rep, 2022. 71(44): p. 1418–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.World Health Organization Global Polio Eradication Initiative. Interim guidance on the use of novel oral polio vaccine type 2 (nOPV2) for the response to type 2 circulating vaccine-derived poliovirus (cVDPV2) during the initial use period (Addendum to the standard operating procedures for response to a poliovirus event or outbreak, version 3.1). 2020. Nov 7, 2022]; Available from: https://polioeradication.org/wp-content/uploads/2020/11/EN-interim-Guidance-on-the-use-of-nOPV2-during-the-initial-use-period-Oct2020.pdf. [Google Scholar]
- 88.Thompson KM and Kalkowska DA, Potential Future Use, Costs, and Value of Poliovirus Vaccines. Risk Anal, 2021. 41(2): p. 349–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fortner R, Has the billion dollar crusade to eradicate polio come to an end? BMJ, 2021. 374: p. n1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Duintjer Tebbens RJ and Thompson KM, Poliovirus vaccination during the endgame: insights from integrated modeling. Expert Rev Vaccines, 2017. 16(6): p. 577–586. [DOI] [PubMed] [Google Scholar]


