Figure 2: The LFA-1-ICAM axis in distinct phases of naïve murine T cell priming and differentiation in reactive lymph nodes.
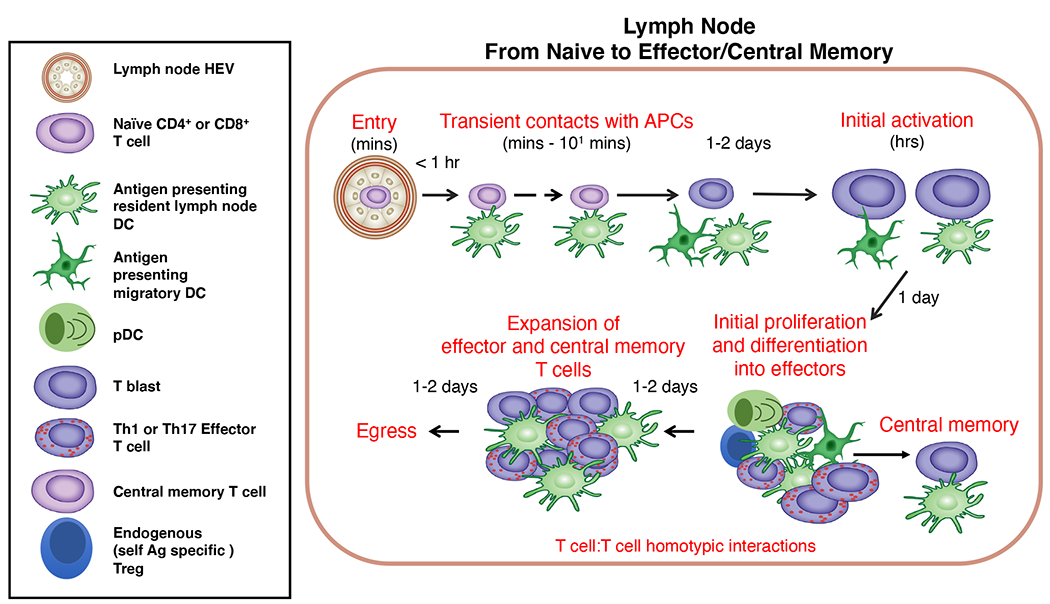
T cells use their LFA-1 to enter lymph nodes by arresting on ICAM-1 and ICAM-2 expressed by HEVs in response to chemokine signals (not shown). Initial naïve T cell activation by antigenic signals takes place by ICAM-1-independent serial encounters with LN and migratory DCs that present cognate antigenic peptide/MHC complexes following immunization or infection[29, 35]. During this phase the antigen-stimulated T cells undergo sequential activation switches that render their LFA-1 sensitive to inside-out activation by additional TCR signals. In phase 2, stable T-DC conjugates lasting for several hours take place and are mediated by DC ICAM-1[35]. Later on the daughter T cells use their LFA-1 to interact with ICAMs clustered on other daughter T cells, while encountering additional antigenic and co-stimulatory signals from resident DCs, migratory DCs and plasmacytoid DCs [42]. These signals are likely needed for T cell differentiation into effector T cells and central memory T cells and are ideally transmitted within LFA-1-stabilized immune synapses. DCs engaged by cognate antigen-activated T cells can also serve as bridges between TCR activated CD4+ and TCR activated CD8+T cells and these clusters may also recruit polyclonal Tregs with highly activated LFA-1 to their vicinity [140]. These Tregs may help attenuate excessive T cell proliferation and differentiation. The extent of T cell differentiation depends on the type of pathogen, the distribution of antigens on distinct subsets of DCs, and may vary with the type of draining lymph node.
