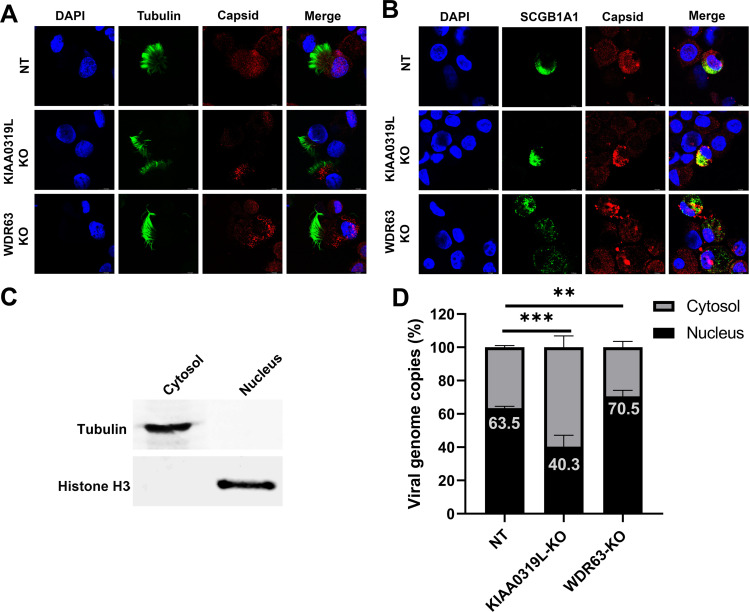
Fig 9.
Localization of the AAV2.5T capsid in transduced HAE-ALI cultures. AAV2.5T was used to apically transduce HAE-ALI cultures as indicated at an MOI of 20,000. (A and B) rAAV2.5T capsid staining. At 3 days post-transduction, cells were digested off the transwell membranes and cytospun onto slides. The slides were fixed and permeabilized, followed by co-immunostaining of the ciliated cell marker (tubulin, panel A) or club cell marker (SCGB1A1, panel B) with the AAV2.5T capsid. The stained cells were subjected to imaging under confocal microscopy at a magnification of ×100 (CSU-W1 SoRa, Nikon). (C and D) rAAV2.5T genome distribution. At 3 days post-transduction, cells in the transwell were washed with Accutase three times and then dissociated by incubation with Accutase for 1 hour. After washing, a cell fraction extraction kit (Thermo Fisher) was used to extract the cytosol and nucleus. (C) Western blotting of the cytosol and nucleus fractions extracted from the non-target control HAE-ALI cultures using anti-tubulin and anti-histone H3, respectively. (D) Quantification of the viral genome in each fraction. Viral DNA was extracted from each fraction and quantitated using qPCR. Error bars represent the standard error of the mean (SEM) from three transwell replicates.