Abstract
Introduction: The application of Zinc oxide nanoparticles (ZnO NPs) is substantially growing in industrial products. Therefore, humans are increasingly exposed to ZnO NPs daily due to their extensive range of applications, raising worries about their possible toxicity. Aim: In this study, the ameliorative effects of raw Phoenix dactylifera L. (date palm) pollens (DPP) and Spirulina platensis (SP) independently against ZnO NPS-induced hepatoxicity in male albino rats were examined. Methods: Six groups (6/group) of adult male albino rats received oral treatment using distilled water (control), SP (1000 mg/kg b. wt.), DPP (100 mg/kg b. wt.), ZnO NPs (100 mg/kg b. wt.), ZnO NPs +SP, and ZnO NPs + DPP respectively for 15 days. Results: The results of the biochemical investigation indicated that the administration of ZnO NPs substantially upregulated (p < 0.05) transaminases, alkaline phosphatase, and bilirubin serum levels. Malondialdehyde and pro-inflammatory cytokine serum levels were also elevated after ZnO NPs administration. Simultaneously, the downregulated catalase and glutathione peroxidase serum activities were significantly suppressed in ZnO NPs treated rats. Moreover, exposure to ZnO NPs induced liver histopathological alterations. The administration of SP and DPP ameliorated the aforementioned effects caused by ZnO NPs. This result can be attributable to the downregulation of hepatic transaminases, alkaline phosphatase, and bilirubin in the serum and the antioxidation system's equilibration, thus alleviating the accumulation of reactive oxygen species. Conclusion: SP and DPP are natural antioxidants with the potential to eliminate inflammation as well as oxidative damage caused by ZnO NPs in hepatic tissue.
Keywords: zinc oxide nanoparticles, Spirulina platensis, Phoenix dactylifera L, hepatotoxicity
Graphical abstract
Introduction
Spirulina platensis (SP), referred to as Arthrospira platensis, is a cyanobacterium that grows in alkaline lakes and has been used as animal feed.1 Protein (involving the whole essential amino acids), vitamins (pro-vitamin A, K, C, E, and B12), gamma-linolenic acid, beta carotene, in addition to various active substances like trace elements, phenolic compounds, and phycocyanin are all abundant in SP. It also contains a significant amount of sulfolipids and glycolipids, as well as calcium, iron, magnesium, manganese, zinc, and potassium in terms of mineral content.2–4 Spirulina has anti-bacterial, immunomodulatory, antioxidant, anti-inflammatory, anti-viral, and anticancer properties, as well as beneficial impacts against anemia, diabetes, obesity, malnutrition, hyperlipidemia, and toxicity induced by heavy metals, as demonstrated a number of recent studies.5–8
Spirulina is considered safe for human consumption due to its longstanding reputation as a dietary source, its optimal safety profile in animal and human trials, and its potential for use as both a medicine and a dietary supplement.9 Previous research10 has demonstrated that SP has neuroprotective properties due to its ability to significantly reduce cerebral cortical damage and increase post-stroke locomotor activity in mice. SP has an anti-inflammatory effect on mice with Zymosan-producing arthritis by decreasing the beta-glucuronidase concentration in the diarthrosis fluid. Moreover, SP can reduce the negative effects of acetic acid-induced ulcerative colitis in rats by increasing the activity of antioxidant enzymes catalase (CAT) and superoxide dismutase (SOD), as well as glutathione (GSH) content. This is accompanied by a substantial decrease in lipid peroxidation, which induces malondialdehyde (MDA) and protein carbonyl inclusion to control prostaglandins and pro-inflammatory cytokines.8,11
Another study2 indicated that bioactive chemicals of SP show inhibitory effects against a variety of viruses, including HIV-1, HSV-1, HSV-2, HCMV, influenza type A, and measles. The mean concentration of capsular haemoglobin (MCH) in the blood is increased when SP is administered to anaemic elderly adults. Furthermore, 12 weeks of SP supplementation demonstrated a substantial improvement in the mean concentration of haemoglobin, mean corpuscular volume (MCV), and mean corpuscular haemoglobin (MCH) in children with anaemia. In addition, it increased the iron level in the blood, indicating that SP can be used as an anaemia medicine.
The date palm pollens (DPP) of P. dactylifera L. are native to the Middle East area, and the traditional medicine used DPP to treat a variety of illnesses, including memory loss, inflammation, loss of consciousness, pyrexia, paralysis, as well as numerous nervous malfunctions.12 Because DPP contain some promising phytochemicals, they are an excellent candidate for antioxidant processes13,14 and have been shown to be protective against oxidative damage caused by various toxicants.15–18 DPP also have anti-apoptotic, aphrodisiac, and anti-coccidial properties.19–21 Furthermore, previous research22,23 has shown that DPP can relieve infertility in male rats via gonadotrophic activity as they contain estrogenic compounds such as estradiol, estriol, and estrone.
Clinical studies have revealed that DPP reduce oral mucositis in humans24 and demonstrates anti-inflammatory and antiproliferative properties in rats.19 Acetaminophen (APAP) induced oxidative damage in the experimental animals’ hepatic and renal tissue, which can be prevented by DPP therapy. Biochemical marker regulation may be responsible for the DPP’s antioxidant, membrane-stabilizing, and antihyperlipidemic activities. DPP’s wide range of pharmacological effects is due to their potent and beneficial components, which include phenolics, carotenoids, flavonoids, minerals, vitamins, fatty acids, organic acids, and amino acids, which are responsible for its wide spectrum of pharmacological actions. A recent study demonstrated that25 DPP are ideal for biochemical applications due to their high drug-loading capacity, excellent storage stability, adaptability, biocompatibility, and low toxicity. Curcumin-loaded DPP, for instance, demonstrated potent antibacterial activity against drug-resistant gram-negative bacteria.
Nanotechnology has rapidly advanced in many areas of human life in recent years, including biomedicine, food, industry, agriculture, and cosmetics.26,27 The properties of nanoparticles are primarily determined by their nanoscale structure, structure-related electronic configurations, size, as well as an extremely high surface-to-volume ratio in comparison to bulk materials.28 ZnO NPs are a popular type of nanomaterial. They have distinct properties like antimicrobial and UV absorption, in addition to being commonly utilized in food preservatives, medicinal products, beauty products, and other applications.29 Because of their wide range of applications, humans are increasingly exposed to ZnO NPs in their daily lives, raising concerns about their potential toxicity. Several studies have found that elevated dosage of ZnO NPs can induce apoptosis in mouse model hepatocytes and cause severe oxidative stress.30,31 Early literature has shown that ZnO NPs primarily increase serum urea and creatinine levels in mice by exfoliating renal tubule epithelial cells and contracting and rupturing the glomerulus.32 Furthermore, the carcinogenicity of ZnO NPs, such as invasion, independent migration, and proliferation, was assessed.33 A previous study34 demonstrated that ZnO NPs have the potential to induce apoptosis in testicular cells, potentially affecting spermatogenesis and thus male fertility.
Currently, accumulated data indicate that certain natural antioxidants and vitamins have a promising protective effect against multiple types of nanoparticle damage. The ingestion of ZnO NPs has been associated with severe liver damage, and vitamin E pretreatment has been suggested to mitigate some of these adverse effects.35 Furthermore, royal jelly was more potent and effective as an antioxidant than molybdenum nanoparticles (MoO3 NPs)-induced oxidative damage.36 Previous research found that melanin, α-lipoic acid, and quercetin have hepatoprotective properties in gold nanoparticles (Au NPs) -treated rats, which has been linked to the reduction of lipid peroxidation.37 Furthermore, it has been demonstrated that a combination of vitamin E and -lipoic acid can reduce pathological kidney damage in rats treated with Au NPs by inhibiting lipid peroxidation and increasing GSH levels.38
Based on the previous findings, the potential benefits of SP and DPP effects against ZnO NPs -induced hepatotoxicity have not yet been demonstrated. Therefore, in this study, we aimed to evaluate the toxic effects of the administration of ZnO NPs on liver structure and function. In addition, we examined whether treatment with SP and DPP could afford ameliorative effects through their antioxidant and anti-inflammatory properties. Our proposed protocol in the current study involves the evaluation of (i) ZnO NPs characterization; (ii) hepatic injury biomarkers; (iii) oxidative stress and antioxidant markers; (iv) pro-inflammatory factors; and (v) histopathological analysis.
Material and methods
Tested chemicals
The ZnO NPs (20–60 nm, >98% pure) were obtained from Nano Gate Company, Egypt. Dispersion of powdered ZnO NPs was done in deionized water, and the suspension was ultrasonicated for 20 min for all experimental work to ensure complete dispersion (Ivymen ultrasonic homogenizer, model CY-500, Spain). Before using the ZnO suspension, it was vortexed for 1 min.27,39 The Spirulina powder, Spirulina platensis, and raw date palm, P. dactylifera L., pollens powder were obtained from IMTENAN company, Egypt. The chemical composition of SP includes 50%–75% high-value proteins, the richest sources of iron, carotene (vitamin A), antioxidants, minerals, and essential fatty acids. DPP (100% Natural) are a source of proteins, vitamins (B2, B6, C), and minerals (iron, zinc). They were suspended in distilled water for oral administration in rats in accordance with their body weight.
Characterization of ZnO NPs
Particle size analysis and zeta potential were determined as follows: before analysis, 10 mg of the sample was dispersed in deionized water (1 mL) and sonicated (Ivymen ultrasonic homogenizer, model CY-500, Spain) for 5 min. Dynamic Light Scattering (DLS) was utilized for the analysis of the size distribution and particle size with respect to polydispersity index (PDI) and diameters of average volume utilizing (Zetasizer Nano ZN-Malvern Panalytical Ltd-United Kingdom) at 25 ° C, with a 173° fixed angle. The samples were examined in triplicate. The same equipment was utilized to calculate zeta potential.
Fourier transformation infrared spectroscopy (FT-IR): the prepared particles’ FT-IR were obtained on a Thermoscientific-Nicole IS 10 (USA). Pellet preparation was done via grinding ZnO NPs (2–6 mg) with potassium bromide (180 mg). Tablets were prepared before being fixed on the holder for examination. The spectra were scanned over the wave number range of 4,000 to 400/cm.
A transmission electron microscope (TEM) for nanoparticles was utilized to determine the shape and size of the prepared nanoparticles (JEM-100CXII, Japan). Therefore, an aqueous dispersion of the NPs was dropped on a carbon-coated copper grid and allowed to evaporate.
Animal and experimental design
The Laboratory Animal Centre provided 36 adult male Sprague-Dawley rats (weighing 150–200 g). They were housed in stainless-steel cages (6 rats/cage) and left for acclimatization at 25 ± 3 °C and a 12/12 h natural daylight cycle two weeks before the experiment. Throughout the study, the animals had free access to water and food. The Commission on the Ethics of Scientific Research (Faculty of Pharmacy, Minia University) approved the study protocol (Code number of the Project: MPEC230511).
The rats were assigned to six groups (six animals each) as follows:
Group 1 (control group): rats received an equal volume of distilled water via oral gavage for two weeks.
Group 2 (SP treated group): rats were treated with SP (1,000 mg/kg b. wt.)40,41 via oral gavage for two weeks.
Group 3 (DPP treated group): rats were treated with DPP (100 mg/kg b. wt.)42 via oral gavage for two weeks.
Group 4 (ZnO NPs treated group): rats were treated with ZnO NPs (100 mg/kg b. wt.) via oral gavage for two weeks. According to previous studies,43,44 the ZnO NPs dose was chosen because it was found to induce hepatic impairment, as indicated by physiological biomarkers, histological structures of rats’ liver tissues, and oxidative stress markers.
Group 5 (ZnO NPs + SP group): rats were treated with ZnO NPs (100 mg/kg b. wt.) and followed by 1,000 mg/kg b. wt. of SP after each other via oral gavage for two weeks.
Group 6 (ZnO NPs + DPP group): rats were treated with ZnO NPs (100 mg/kg b. wt.) and followed by 100 mg/kg b. wt. of DPP after each other via oral gavage for two weeks.
Measurement of body weights
The body weight of each rat from every experimental group was estimated initially and before dissection. All rats were weighed using an automatic GX-600 balance (A&D Company, Ltd, Tokyo, Japan).
Collection of samples
At the conclusion of the experiment, all animals were sacrificed under diethyl ether anesthesia while fasting, and blood samples were collected. The blood samples were then allowed to clot by being left undisturbed at room temperature for 15–30 min before centrifugation at 2,683 × g for 10 min (4 °C). The resultant supernatant is referred to as serum. The serum was carefully poured into a new sterilized tube utilizing a sterile pipette to avoid contamination by residual red cells at the bottom of the tube. Subsequently, sera were collected and kept at −80 °C for further biochemical analysis. Following blood sampling, the liver tissues from each animal were separated. Approximately, one gram of liver tissues were washed three times with cold NaCl solution (0.9%) before being homogenised (SONOBIO Handheld Ultrasonic Homogenizer, China) in cold phosphate-buffered saline (PBS), pH 7.4.45 The liver homogenates were centrifuged for approximately 15 min at 1509 × g at 4 °C, and the supernatants were cautiously separated and kept at −80 °C to be used for liver bioassay. Small portions from liver tissues were immersed in 10% formaldehyde for histopathology.
Biochemical assays
The alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) levels were determined calorimetrically by spectrophotometer using commercial kits (Biosystems S.A., Barcelona, Spain) in accordance with to methods of previous studies.46–48 According to the ALT assay kit’s principles technique, ALT catalyses the transfer of the amino group from alanine to 2-oxoglutarate, resulting in pyruvate and glutamate. The catalytic concentration is calculated using the lactate dehydrogenase (LDH) coupled reaction and the rate of reduction of NADH at 340 nm. In the AST test kit’s principles method, AST catalyses the transfer of the amino group from aspartate to 2-oxoglutarate, leading to formation of oxalacetate and glutamate. The concentration is calculated using the malate dehydrogenase (MDH) coupled reaction and the rate of decrease of NADH at 340 nm. In the ALP kit assay, ALP catalyses the transfer of the phosphate group from 4-nitrophenylphosphate to diethanolamine (DEA) in alkaline medium releasing 4-nitrophenol. The rate of 4-nitrophenol production at 405 nm is used to calculate the catalytic concentration. ALT, AST, and ALP levels were expressed as U/L.
Serum albumin level was assessed by Webster et al.49 calorimetrically using commercial diagnostic kits obtained from Spinreact, Girona, Spain. Briefly, in this procedure, albumin in the presence of bromcresol green at a slightly acidic pH causes the indicator’s colour to change from yellow-green to green-blue. The intensity of the colour produced is related to the concentration of albumin in the sample. Total bilirubin levels in the serum samples were determined according to a previous study50 using commercial kits (Vitro Scient, Giza, Egypt). Bilirubin quantification depends on the chemical reaction between bilirubin and diazotised sulfanilic acid. Only direct conjugated bilirubin will react in this way in aqueous solution. To estimate total bilirubin, however, unconjugated bilirubin must be released from albumin attachment by becoming water soluble. The levels of albumin and bilirubin were expressed as g/dl.
According to previous researches,51,52 serum malondialdehyde (MDA) was identified as a lipid peroxidation product using thiobarbituric acid-reactive compounds. The absorbance of the resultant pink product can be measured at 534 nm. In addition, glutathione peroxidase (GPx) and catalase (CAT) activities were calorimetrically quantified utilizing Bio-Diagnostic kits, Egypt.53,54 The basic technique of the GPx test kit indicates that the oxidation of NADPH to NADP+ is accompanied by a reduction in absorbance at 340 nm, offering a spectrophotometric way of detecting GPx enzyme activity. According to the manufacturer’s protocols, CAT reacts with a known quantity of H2O2. Catalase inhibitor is used to stop the reaction after exactly one min. In the presence of peroxidase, residual H2O2 interacts with 3,5-Dichloro-2-hydroxybenzene sulfonic acid (DHBS) and 4-aminophenazone (AAP) to produce a chromophore with a colour intensity that is oppositely proportional to the level of catalase in the initial specimen. The results of MDA were reported in nmol/ml, while GPx and CAT results were reported in U/L.
Interleukin- 6 (IL-6) (Cat. No. E-EL-R0015) and serum tumor necrosis factor-alpha (TNF- α) (Cat. No. E-EL-R0019) were determined utilizing specific enzyme-linked immunosorbent assay (ELISA) kits (Elabscience Biotechnology Inc., USA). The concentrations of IL-6 and TNF- α were expressed in pg/ml. All biochemical assays were calculated using the instructions provided by the manufacturer.
Zn concentration in the liver
The previously prepared homogenized liver tissue samples were utilized to estimate the Zn content. This analysis was conducted using a commercial test kit (cat. no. MET-5138, Cell Biolabs, Inc, San Diego, USA). Briefly, the deproteinizing solution in a 1:1 ratio was added to the homogenized hepatic tissue samples. The proteins were immediately precipitated. The samples were mixed thoroughly, incubated for 5 min, and then centrifuged at 14,000 × g for 5–10 min to pellet the precipitate. The liquid was carefully removed for testing, and the supernatant was tested undiluted or diluted in deionized water as needed. Zinc concentrations were determined by comparing sample zinc concentrations to a known zinc standard.
Histopathology
For histological investigation, the rats were dissected, and the liver was immediately taken out. Liver tissues were cut into a thickness of 3–4 mm, dispersed in neutral buffered formalin (10% NBF) before dehydration in a series of ethanol concentrations, and then cleared in xylene before being embedded in paraffin. In order to examine general tissue structure, paraffin samples were sectioned with a microtome at a thickness of 4–6 μm and stained with Hematoxylin and Eosin (H&E). H&E-stained sections were examined utilizing a Leica microscope (CH9435 Hee56rbrugg-Leica Microsystems-Switzerland).
Statistical analysis
The current study’s findings were analyzed utilizing the 22nd version of the SPSS software. A one-way analysis of variance (ANOVA) was utilized to determine significance, followed by a least significant difference LSD as a post hoc test. The results were expressed as the mean ± SE, and the level of significance was set at P < 0.05.
Results
Characterization of ZnO NPs
Particle size analysis and zeta potential
Particle size distribution utilizing a particle size analyzer revealed that the ZnO NPs had a 211.3 ± 3.3 nm mean particle size. Furthermore, the polydispersity index (PDI) was 0.192 (mid-range polydispersity)55 (Fig. 1). The Zeta potential of the ZnO NPs was determined to estimate nanoparticles’ surface charge in solution. The outcomes indicated that the particles were positively charged with a range of (+25.4 ± 0.41 mv) (Fig. 2).
Fig. 1.
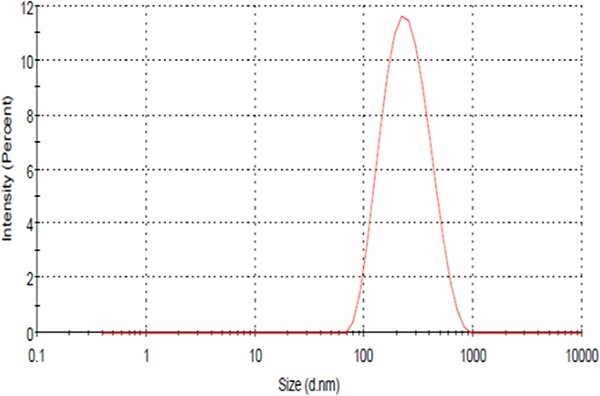
The particle size distribution curve of prepared ZnO NPs.
Fig. 2.

Zeta potential curve of prepared ZnO NPs.
Fourier-transform infrared spectroscopy (FT- IR)
FTIR analysis is helpful in determining the several distinct functional groups and purity of the examined ZnO nanoparticles. The FTIR spectrum of ZnO NPs was carried out within the wavenumber range of 4,000 to 400 cm−1, as shown in Fig. 3. The wide peak observed at 3,440 cm−1 is due to the (O-H) group stretching vibration.56,57 Absorption peaks obtained at 1,632 cm−1 and 1,413 cm−1 are attributable to the stretching vibrations of (C=C) and (C-C), respectively. It is known that the region below 1,000 cm−1 in the FTIR spectrum is considered the fingerprint band for metal oxides.56 The intense peak around 448 cm−1 is a characteristic peak of stretching Zn-O vibration that verifies the existence of ZnO.56,58
Fig. 3.

Fourier-transform infrared spectrum showing the functional groups of prepared ZnO NPs.
Transmission electron microscope (TEM) for nanoparticles
The TEM micrograph shown in Fig. 4 revealed spherical-like structures with a size of 38.24 nm.
Fig. 4.

Electron micrograph showing spherical-shaped particles of around 38.24 nm in diameter.
Morbidity, mortality, and bodyweight
No toxic signs such as diarrhea, hair loss, or mortalities were found in the experimental groups. The body weight of ZnO NPs-treated rats was significantly lower by the end of the experiment than the control, SP, and DPP groups. The body weight of rats in groups ZnO NPs + SP and ZnO NPs + DPP significantly increased when compared to control and ZnO NPs-treated groups (Table 1).
Table 1.
Mean values of initial, final body weights (g) of all experimental rats over an experimental period.
| Parameters | Groups | |||||
|---|---|---|---|---|---|---|
| Control | SP | DPP | ZnO NPs | ZnO NPs + SP | ZnO NPs + DPP | |
| Initial body weight (g) | 141.00 ± 1.23 | 140.83 ± 1.22 | 140.00 ± 1.52 | 155.33 ± 1.28 | 145.83 ± 2.03 | 140.66 ± 1.54 |
| Final body weight (g) | 154.00 ± 1.29 | 159.83 ± 1.88a | 161.33 ± 1.17a | 132.16 ± 1.88b | 168.00 ± 2.39a,b | 169.66 ± 2.51a,b |
Data are expressed as the mean ± SE (n = 6). Significance at P < 0.05.
aSignificantly different from ZnO NPs group.
bSignificantly different from control group.
Serum hepatic injury biomarkers
ALT, AST, ALP, and bilirubin serum levels in the ZnO NPs group were significantly elevated than in the control, SP, and DPP groups (P < 0.05). Furthermore, compared to the ZnO NPs group, the previous parameters were dramatically reduced by SP and DPP treatment in groups ZnO NPs + SP and ZnO NPs + DPP but remained higher than in control, SP and DPP groups (P < 0.05). Only bilirubin showed a non-significant decrease when comparing ZnO NPs + SP and ZnO NPs + DPP to the control, SP, and DPP groups (P < 0.05). Albumin levels were considerably lower in the ZnO NPs group than in control, SP, and DPP (P < 0.05). The albumin serum level improved in the ZnO NPs + SP and ZnO NPs + DPP treated groups compared to the ZnO NPs group (Table 2).
Table 2.
Serum levels of hepatic function biomarkers in the control and experimental groups.
| Parameters | Groups | |||||
|---|---|---|---|---|---|---|
| Control | SP | DPP | ZnO NPs | ZnO NPs + SP | ZnO NPs + DPP | |
| ALT (U/L) | 59.33 ± 3.68 | 45.66 ± 3.29a | 43.00 ± 3.0a | 89.83 ± 1.77b | 78.00 ± 2.42a,b | 74.50 ± 2.65a,b |
| AST (U/L) | 112.50 ± 3.11 | 102.00 ± 3.61a | 90.33 ± 1.45a | 145.83 ± 3.78b | 133.67 ± 2.06a,b | 127.6 ± 2.36a,b |
| ALP (U/L) | 254.67 ± 3.42 | 239.50 ± 2.55a | 231.50 ± 3.25a | 285.67 ± 4.57b | 275.83 ± 2.13a,b | 267.5 ± 2.74a,b |
| Bilirubin (g/dl) | 0.226 ± 0.022 | 0.175 ± 0.011a | 0.146 ± 0.012a | 0.415 ± 0.018b | 0.240 ± 0.013a | 0.210 ± 0.012a |
| Albumin (g/dl) | 2.19 ± 0.12 | 2.93 ± 0.09a | 3.11 ± 0.14a | 1.37 ± 0.05b | 2.41 ± 0.08a | 2.66 ± 0.09a,b |
Data are expressed as the mean ± SE (n = 6). Significance at P < 0.05.
aSignificantly different from ZnO NPs group.
bSignificantly different from control group.
Oxidative stress and antioxidant markers
Serum MDA levels were substantially higher in ZnO NPs-treated rats compared to control, SP, and DPP-treated groups. Furthermore, oral SP and DPP treatment significantly reduced MDA levels in the ZnO NPs + SP and ZnO NPs + DPP groups compared to ZnO NPs (P < 0.05) (Fig. 5A). GPx and CAT activities were considerably reduced in ZnO NPs -treated groups compared to control rats. However, the serum GPx and CAT levels of ZnO NPs in rats treated with SP and DPP showed an improvement (but did not return to normal levels) (Figs. 5B and C).
Fig. 5.
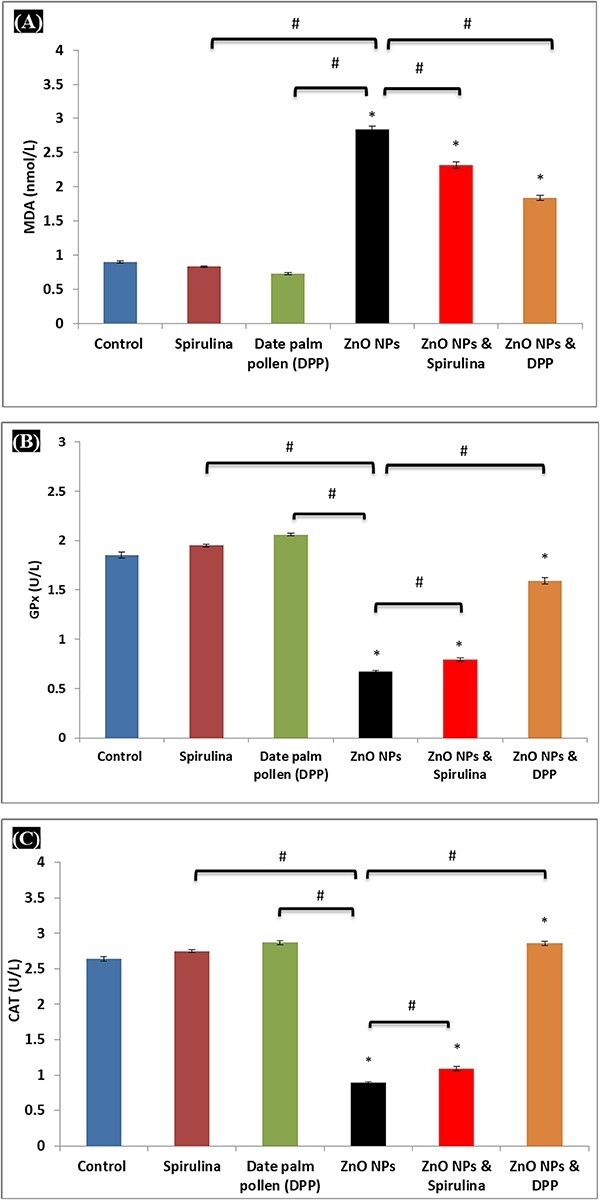
A) Levels of MDA, B) GPx, and C) CAT at the end of the treatment period. Results are expressed as (mean ± S.E, n = 6), significance at P < 0.05, * indicates significance from the control group (P < 0.05) and # indicates significance from the ZnO NPs group (P < 0.05).
Pro-inflammatory factors
Figure 6A and B shows that ZnO NPs group has higher levels of TNF-α level and IL-6 than control, SP, and DPP-treated groups (P < 0.05). In contrast, intoxicated rats treated with SP and DPP had lower TNF-α and IL-6 levels, though they remained more elevated than those in control, SP, and DPP groups (P < 0.05).
Fig. 6.

A) Levels of TNF-α and B) IL-6 at the end of the treatment period. Results are expressed as (mean ± S.E, n = 6), significance at P < 0.05, * indicates significance from the control group (P < 0.05) and # indicates significance from the ZnO NPs group (P < 0.05).
Zn concentration in the liver
The Zn levels in liver tissues were considerably higher in the ZnO NPs-treated group compared to the other treatment groups (P < 0.05). However, treatment with SP and DPP showed improvement in the Zn liver concentrations in the ZnO NPs + SP and ZnO NPs + DPP groups (but not the return to their normal level) (Table 3).
Table 3.
Zn concentrations in liver tissues (μM) in the control and experimental groups.
| Parameter | Groups | |||||
|---|---|---|---|---|---|---|
| Control | SP | DPP | ZnO NPs | ZnO NPs + SP | ZnO NPs + DPP | |
| Zn concentrations (μM) | 0.81 ± 0.009 | 0.74 ± 0.009a | 0.79 ± 0.016a | 2.95 ± 0.031b | 1.79 ± 0.016a,b | 1.83 ± 0.062a,b |
Data are expressed as the mean ± SE (n = 6). Significance at P < 0.05.
aSignificantly different from ZnO NPs group.
bSignificantly different from control group.
Histopathological analysis
The sections of hepatic tissues from control, SP, and DPP-treated rats demonstrated the standard architecture of liver tissue assembled in the normal central vein with intact lining endothelium and normal hepatic cords and integral blood sinusoids (Figs. 7A–C).
Fig. 7.
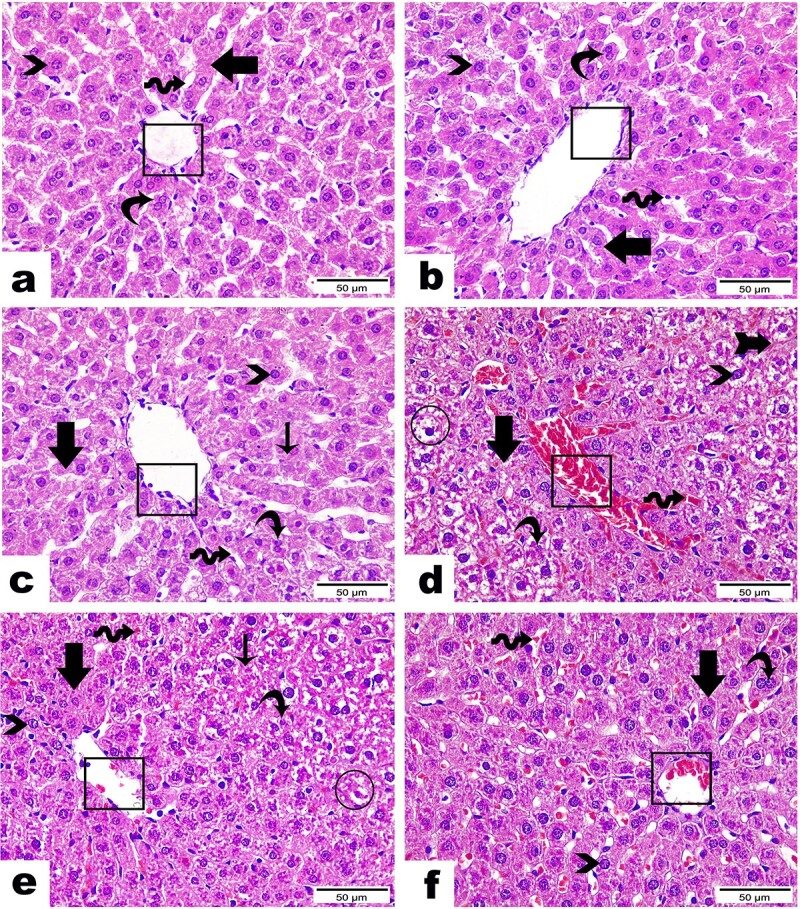
Photomicrographs of the histopathological variations in liver tissue sections between groups (H & E Stain, magnification power = ×400 and scale bar = 50 μm): Liver sections from A) control group, B) SP group, and C) DPP group demonstrating the standard architecture of liver tissue assembled in a normal central vein (cubes), regular hepatic cords (arrows), uninuclear (arrowheads) or binuclear (curvy arrows) hepatocytes, in addition to integral blood sinusoids (wave arrows). Liver section from D) ZnO NPs group showing severe congestion in central vein (cube). Congested blood sinusoids (wave arrow); others were marked with a collapsed lumen (arrow with tail). Hepatic cords lost their organization (arrow) and emphasized hepatocellular hypertrophy and hydropic degeneration (circle) along with either an intact nucleus (arrowhead) or an apoptotic one (curvy arrow). Liver section from E) ZnO NPs + SP group revealed moderate improvement, as demonstrated by the absence of congestion in central veins (cube) while detected in low amounts along blood sinusoids (wave arrow), moderate hepatocellular degeneration (circle) with cytoplasmic vacuolation and pyknotic nuclei (curvy arrow) in addition to other areas marked normal architecture with intact hepatocyte (arrowhead). Some hepatic cords were marked in a regular manner (thick arrow), but few still lost their organization (thin arrow). Liver section from F) ZnO NPs + DPP group displaying a remarkable recovery proven by apparently normal hepatocytes (arrow) with vesicular nuclei and prominent nucleoli, either mono (arrowhead) or binucleated one (curvy arrow). In contrast, central vein (circle) as well as blood sinusoids (wave arrow) are still noticed with congestion.
In contrast, hepatic sections in the ZnO NPs group highlighted serious hepatic injuries, such as severe congestion of blood sinusoids and the central veins. Additionally, hepatic cords lost their structure with hepatocellular hypertrophy and hydropic degeneration (Fig. 7D).
The group treated with ZnO NPs + SP exhibited substantially improved histopathological changes than the ZnO NPs treated group. Figure 7E depicts the absence of congestion in the central vein while it is detected in small amounts along the blood sinusoids, as well as moderate hepatocellular degeneration with cytoplasmic vacuolation. In addition, other areas displayed normal architecture with intact hepatocytes were observed. Moreover, some hepatic cords were marked in a regular pattern, but a few remained disorganized. Similarly, tissue sections from the ZnO NPs + DPP group displayed a remarkable recovery, as evidenced by the presence of apparently normal hepatocytes, whereas the central vein and blood sinusoids remained congested (Fig. 7F).
Discussion
Nanoparticles and nanotechnology could be a serious concern in toxicological processes. Due to the prevalent utilization of ZnO NPs in household products, medicine, agriculture, and the food industry, the human body can be exposed to nanoparticles via dermal penetration, intravenous injection, inhalation, and oral ingestion. One of the most significant routes for nanoparticle absorption is the gastrointestinal tract.59 The toxic ZnO NPs impacts were proven to be linked to their nano properties.60 Compared to traditional ZnO, the ZnO NPs exhibited various physicochemical characteristics in terms of electrostatic interaction, protein crown binding, shape, surface modification, and specific surface area.61 Because of their small size, nanoparticles can be distributed to various body regions when ingested. They have the ability to cross the intestine and spread to the stomach, intestine, liver, spleen, kidney, heart, lung, and blood.62 Previous study63 has described bulk and ZnO nano particles’ acute oral toxicity in female Wistar rats. They discovered that NP absorption is determined to be 15–250 times greater than that of bulk particles. Also, this study measured the bioavailability of Zn in plasma and found that the NP-treated group absorbed a greater total amount of ZnO into their blood than the control group.
Concerning the characterisation of ZnO NPs (Figs 1–4), TEM revealed spherical-shaped particles with an approximate 38.24 nm diameter. The Fourier-transform infrared spectrum showed peaks at 3,440 cm−1, 1,632 cm-1, and 1,413 cm−1 for the (O-H) group, (C=C) stretching, and (C-C) stretching vibrations, respectively. The size of ZnO NPs determined by DLS in this study was 211.3 ± 3.3 nm, indicating an increase in size over TEM analysis. These observed size differences were primarily due to agglomeration property in suspension.
The current study’s findings on body weight decline align with Hong et al.64 who discovered that rats receiving different doses of ZnO NPs via gavage demonstrated lower body weight due to decreased food consumption. This result suggests that exposure to ZnO NPs has a significant impact on animal health.
Our findings showed substantially elevated ALT and AST levels following ZnO NPs treatment (group 4). This observation agreed with several previous reports,44,63,65 which indicated that the increased level of these enzymes in serum could be due to hepatocyte cell injuries. Zn2+ ions release is predominantly responsible for ZnO toxicity. ZnO NPs are mainly dissolved inside the stomach, releasing Zn2+ ions that tissues take up through interactions between Zn and ligands that contain sulfur in proteins.30 Zn2+ ions may influence hepatocyte cells’ membrane permeability in the liver since membrane proteins are cation-binding proteins with cysteine-rich residues that can result in a Zn-S bond. If released, Zn2+ might combine with membrane-bound enzymes through the reactions of the sulfhydryl group to generate stable mercaptides, or it could quickly bind to cell membranes, as evidenced by its high affinity for phospholipids.66 Consequently, Zn-S bond formation by a membrane protein might alter the permeability of cell membranes.
In the present study, ALP serum levels in the ZnO NPs group were substantially elevated than in the controls. This finding is consistent with prior studies that utilized the exact doses in male rats,44,65 denoting that the nanoparticles induce hepatic damage. Albumin, the most prevalent protein in serum, exhibits antioxidant properties, and any decline in this protein can reduce the regulation of cellular oxidative stress.67 In the present study, ZnO NP-treated rats exhibited hypoalbuminemia compared to controls. This outcome is supported by the previous results of Shaban et al.,68 who reported that 40 ppm ZnO-NPs treatment induced a noticeable elevation in albumin level due to either declined hepatic function or elevated oxidation by free radicals caused by ZnO NPs.69
In this study, ZnO NPs’ potential to cause oxidative stress was examined via measurement of serum CAT, GPx, and MDA. It was found that ZnO NPs’ oral administration upregulated MDA levels while downregulating CAT as well as GPx activity. It has previously been demonstrated that ZnO NPs administration results in the generation of highly reactive oxygen species like hydroxyl radicals, superoxide anions, and hydrogen peroxide, inducing oxidative stress in animal cells.70 This finding aligns with prior research,65 which demonstrated that ZnO NPs at concentrations over 50 mg/kg body weight resulted in marked oxidative stress in adult male Wistar rats. Likewise, Attia et al.71 illustrated that daily oral exposure to 40 and 100 mg/kg of ZnO NPs for seven days induced substantial CAT, SOD, and GSH depletion, denoting that ZnO NPs can damage the antioxidant system in brain tissue, and thus causing nitrosative and oxidative stress.
Reactive oxygen species (ROS) are proven to collaborate to release pro-inflammatory mediators,72,73 which is consistent with our findings that the oxidative stress following exposure to 100 mg/kg of ZnO NPs upregulated pro-inflammatory cytokines TNF-α and IL6 concentrations. A previous study71 reported that TNF-α and IL-1β levels in the brain were significantly upregulated, as were apoptotic markers, in groups that received ZnO NPs (100 mg/kg) for seven days. Furthermore, Fujihara et al.74 demonstrated that ZnO NPs resulted in a pro-inflammatory response in vivo, which can be linked to oxidative stress. In addition, hepatic damage was observed at an early stage.
The results revealed significantly higher zinc ions concentration in the hepatic tissues in the ZnO NPs group compared to the control group. Similar to our results, Mohamed et al.44 reported the accumulation of Zn in the hepatic tissues of rats treated with 100 mg/kg ZnO NPs. Furthermore, a previous study75 reported that rats inhaled 20 nm ZnO NPs (2.5 mg/kg bw) twice daily for three days, which increased the zinc content in both the liver and kidneys and caused tissue damage. The current findings demonstrated a significant correlation between intracellular Zn2+ ions accumulation caused by ZnO NPs dissolution and ZnO NPs hepatotoxicity, characterized by increased lipid peroxidation and oxidative stress.
Histopathological changes in the liver were further evidenced by biochemical investigations. In this study, the control, DPP, and SP-treated groups demonstrated normal liver tissues, whereas the ZnO NPs-treated groups exhibited substantial liver damage, demonstrated by central vein as well as blood sinusoid congestion. Additionally, hepatic cords lost their organization with hepatocellular hypertrophy and hydropic degeneration.
Similar results have been reported in rats who received ZnO NPs daily at a 100 mg/kg dose for ten days.44 Previous research76,77 revealed that hepatocyte swelling suggests that the nanoparticles might impact cell membrane permeability in hepatocytes, increasing intracellular water permeability as well as a massive influx of Na ions and water. Moreover, hepatocyte degeneration may be associated with lysosomal hydrolytic enzyme leakage, upregulating cytoplasmic degeneration.78 Further studies79,80 indicated that ZnO NPs might be toxic by accumulating in the liver and inducing intracellular ROS production. Conversely, upregulated ROS levels downregulated mitochondrial membrane potential (MMP) and upregulated apoptotic protein, Bax.
Recent research has focused on the effectiveness of natural antioxidants against ZnO NPs toxicity in rats.35,43 To our knowledge, this is the first study to examine the impacts of SP and DPP on markers of liver histopathology, inflammation, as well as oxidative stress associated with ZnO NPs hepatotoxicity. Our results indicated that the ZnO NPs + SP group gained weight compared to the ZnO NPs group. A previous study3,4 reported that SP has a high nutritional value, with a significant content of minerals, proteins, amino acids, vitamins, and antioxidants, combined with its bioavailability, which contributed to the increase in body weight in the SP group.
Notably, the serum hepatic injury biomarkers were downregulated following SP administration (group 5). These outcomes coincide with those of a number of trials in various animal models that consistently demonstrated the activity of SP as partially or totally normalizing total bilirubin, ALP, AST, and ALT levels.81,82
Moreover, SP administration in rats (group 5) enhanced serum albumin levels. This hepatoprotective impact was attributable to the SP alga’s main antioxidant protein, phycocyanin, which can eliminate hydroxyl-, and peroxyl-free radicals while downregulating nitrite production as well as hindering hepatic lipids peroxidation.83 Multiple studies indicated that phycocyanin also elevated antioxidant activity and albumin bioavailability.67,84,85
These findings prove that SP can control ZnO NPs-induced oxidative stress in rats by restoring CAT and GPx activity while decreasing MDA. Consistent with this hypothesis, prior studies81,82,86 revealed that SP could protect against various toxic substances by upregulating the activity of various antioxidant enzymes, downregulating free radicals, and thus enhancing liver function. These properties can be due to the elevated phenolic compound concentrations, Omega-6, as well as Omega-3 fatty acids detected in SP. These substances have several double bonds in their molecular structure, which are responsible for antioxidant impacts.87
In the present study, rats treated with ZnO NPs + SP group had downregulated TNF-α and IL6 levels compared to ZnO NPs. These findings are consistent with other studies,88,89 which reported that SP exerts various anti-inflammatory and immunomodulatory activities via pro-inflammatory cytokine regulation. SP phycocyanin has been reported to have immunomodulatory activity through stimulating antibody production and regulating gene expression encoding IL-2, IL-1, IL-4, IL-10, IL-6, IL-17, and TNF-α.8 Our histopathological results showed the ability of SP to preserve the hepatocellular membrane’s structural integrity following the elimination of free radicals generated during intoxication by various toxic substances.81,90,91
The current investigation also found that the body weights of ZnO NPs + DPP treated rats increased when compared to ZnO NPs animals. DPP were high in nutrients like minerals, vitamins, amino acids, and phenolic and flavonoid components, contributing to the treatment group’s weight gain.92
The current investigation found that DPP can provide significant hepatic protection against ZnO NPs toxicity. Interestingly, the current study’s findings showed that giving DPP to group 6 reduced the hepatic injury and oxidative damage caused by ZnO NPs. Similarly, a prior study13 found that DPP extract (150 mg/kg) considerably reverses the increases in liver enzymes as DPP prevented the adverse impacts of altered thyroid hormone levels.
Other researchers found that DPP contain antioxidant phytochemicals such as rutin, flavonoids, and phenolic compounds; these phytochemicals were also proven to have hepatoprotective properties in animal models.92–94 Interestingly, DPP therapy (40 mg/kg) was recently found to reduce the negative effects of cadmium-induced testicular damage by prohibiting increased oxidative stress.22
Furthermore, previous research42 supports DPP’s liver damage repair and hepatoprotective properties, where pretreatment with DPP aqueous suspensions (50 and 100 mg/kg b.w.) offer protective effects against Acetaminophen-intoxicated rats due to its potent antioxidant and free-radical scavenging properties.
In the current investigation, DPP were found to have anti-inflammatory properties to some extent. Fatani et al.95 found that suspension or extract of DPP reduced prostatic hypertrophy and lowered the production of pro-inflammatory cytokines in Wistar rats. Furthermore, bioactive DPP components are the key to DPP anti-inflammatory activities in animal models.96,97
Histopathology findings in the current study validated DPP’s protective efficacy against ZnO NPs-induced liver damage. This beneficial effect could be attributed to enhancing the activity of the antioxidant defense system and scavenging ROS. Previous research22 revealed that the antioxidant capabilities of DPP extract could be used to treat cadmium-induced severe testicular lesions. According to Mohamed et al.,14 DPP at a dose of 100 mg/kg may have a possible anti-diabetic effect, where DPP completely normalized diabetic rats’ pancreatic islets and have a therapeutic protective activity by alleviating pancreatic beta cell damage as well as oxidative stress, which can be linked to their antioxidative possibilities.
Conclusion
The current study’s statistical analysis revealed that the ZnO NPs + SP and ZnO NPs + DPP treatment groups improved biochemical parameters and liver histology to some extent compared to the ZnO NPs group. However, when compared to the control group, these groups exhibited only a slight improvement since their values did not reach the control levels. Suggesting varied and higher dosages of SP and DPP is required, as well as longer-term study. Furthermore, future work is needed to determine the efficacy of the co-administration of SP and DPP on hepatotoxicity caused by ZnO NPs.
Supplementary Material
Acknowledgement
The authors are immensely thankful to Prof. Amro K. F. Dyab, Department of Chemistry, School of Sciences and Humanities, Nazarbayev University, Kazakhstan, for his support in analyzing the characterization section of ZnO NPs.
Contributor Information
Diaa B Al-Azhary, Zoology Department, Faculty of Science, Minia University, Cairo-Aswan Road, Minia 61519, Egypt.
Samar A Sawy, Zoology Department, Faculty of Science, Minia University, Cairo-Aswan Road, Minia 61519, Egypt.
Hanaa Fawzy Hassan, Zoology Department, Faculty of Science, Minia University, Cairo-Aswan Road, Minia 61519, Egypt.
Noha M Meligi, Zoology Department, Faculty of Science, Minia University, Cairo-Aswan Road, Minia 61519, Egypt.
Author contributions
Diaa B. Al-Azhary (Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Writing—original draft, Writing—review & editing, Supervision). Samar A. Sawy (Investigation, Resources, Formal analysis, Visualization, Validation), Hanaa Fawzy Hassan (Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing—review & editing, Supervision, Noha M. Meligi (Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Writing—original draft, Writing—review & editing, Supervision
Funding
This research received no external funding.
Conflict of interest statement
None declared.
Data availability
All date related to this article are included in the maintext.
References
- 1. Ouhtit A, Ismail MF, Othman A, Fernando A, Abdraboh ME, El-Kott AF, Azab YA, Abdeen SH, Gaur RL, Gupta I, et al. Chemoprevention of rat mammary carcinogenesis by spirulina. Am J Pathol. 2014:184(1):296–303. [DOI] [PubMed] [Google Scholar]
- 2. Anvar AA, Nowruzi B. Bioactive properties of spirulina: A review. Microb Bioact. 2021:4(1):134–142. [Google Scholar]
- 3. Hoseini SM, Khosravi-Darani K, Mozafari MR. Nutritional and medical applications of spirulina microalgae. Mini Rev Med Chem. 2013:13(8):1231–1237. [DOI] [PubMed] [Google Scholar]
- 4. Soni RA, Sudhakar K, Rana RS. Spirulina – from growth to nutritional product: a review. Trends Food Sci Technol. 2017:69:157–171. [Google Scholar]
- 5. Prabhu S, Vijayakumar S, Praseetha P. Cyanobacterial metabolites as novel drug candidates in corona viral therapies: a review. Chronic Dis Transl Med. 2022:8(3):172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Braune S, Krüger-Genge A, Kammerer S, Jung F, Küpper JH. Phycocyanin from Arthrospira platensis as potential anti-cancer drug: review of in vitro and in vivo studies. Clin Hemorheol Microcirc. 2021:11(2):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kasbi-Chadli F, Coué M, Aguesse A, Grit I, Souque T, Ferchaud-Roucher V, Ouguerram K. Spirulina liquid extract prevents metabolic disturbances and improves liver sphingolipids profile in hamster fed a high-fat diet. Eur J Nutr. 2021:60(8):4483–4494. [DOI] [PubMed] [Google Scholar]
- 8. Wu Q, Liu L, Miron A, Klímová B, Wan D, Kuča K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: an overview. Arch Toxicol. 2016:90(8):1817–1840. [DOI] [PubMed] [Google Scholar]
- 9. Karkos PD, Leong SC, Karkos CD, Sivaji N, Assimakopoulos DA. Spirulina in clinical practice: evidence-based human applications. Evid Based Complement Alternat Med. 2011:2011:531053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. El-Sayed E-S, Hikal M, El-Khair BA, El-Ghobashy R, El-Assar A. Hypoglycemic and hypolipidemic effects of spirulina platensis, phycocyanin, phycocyanopeptide and phycocyanobilin on male diabetic rats. Arab Univ J Agric Sci. 2018:26(3):1121–1134. [Google Scholar]
- 11. Nowruzi B, Sarvari G, Blanco S. The cosmetic application of cyanobacterial secondary metabolites. Algal Res. 2020:49:101959. [Google Scholar]
- 12. Biglari F, AlKarkhi AFM, Easa AM. Antioxidant activity and phenolic content of various date palm (Phoenix dactylifera) fruits from Iran. Food Chem. 2008:107(4):1636–1641. [Google Scholar]
- 13. El-Kashlan AM, Nooh MM, Hassan WA, Rizk SM. Therapeutic potential of date palm pollen for testicular dysfunction induced by thyroid disorders in male rats. PLoS One. 2015:10(10):1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mohamed NA, Ahmed OM, Hozayen WG, Ahmed MA. Ameliorative effects of bee pollen and date palm pollen on the glycemic state and male sexual dysfunctions in streptozotocin-induced diabetic wistar rats. Biomed Pharmacother. 2018:97:9–18. [DOI] [PubMed] [Google Scholar]
- 15. Abdelaziz DHA, Ali SA. The protective effect of Phoenix dactylifera L. seeds against CCl4-induced hepatotoxicity in rats. J Ethnopharmacol. 2014:155(1):736–743. [DOI] [PubMed] [Google Scholar]
- 16. El Arem A, Saafi EB, Ghrairi F, Thouri A, Zekri M, Ayed A, Zakhama A, Achour L. Aqueous date fruit extract protects against lipid peroxidation and improves antioxidant status in the liver of rats subchronically exposed to trichloroacetic acid. J Physiol Biochem. 2014:70(2):451–464. [DOI] [PubMed] [Google Scholar]
- 17. Salem GA, Shaban A, Diab HA, Elsaghayer WA, Mjedib MD, Hnesh AM, Sahu RP. Phoenix dactylifera protects against oxidative stress and hepatic injury induced by paracetamol intoxication in rats. Biomed Pharmacother. 2018:104:366–374. [DOI] [PubMed] [Google Scholar]
- 18. Saafi EB, Louedi M, Elfeki A, Zakhama A, Najjar MF, Hammami M, Achour L. Protective effect of date palm fruit extract (Phoenix dactylifera L.) on dimethoate induced-oxidative stress in rat liver. Exp Toxicol Pathol. 2011:63(5):433–441. [DOI] [PubMed] [Google Scholar]
- 19. Elberry AA, Mufti ST, Al-Maghrabi JA, Abdel-Sattar EA, Ashour OM, Ghareib SA, Mosli HA. Anti-inflammatory and antiproliferative activities of date palm pollen (Phoenix dactylifera) on experimentally-induced atypical prostatic hyperplasia in rats. J Inflamm. 2011:8(1, 1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abedi A, Parviz M, Karimian SM, Rodsari HRS. Aphrodisiac activity of aqueous extract of Phoenix dactylifera pollen in male rats. Adv Sex Med. 2013:03(1):28–34. [Google Scholar]
- 21. Metwaly MS, Dkhil MA, Al-Quraishy S. Anti-coccidial and anti-apoptotic activities of palm pollen grains on Eimeria papillata-induced infection in mice. Biologia. 2014:69(2):254–259. [Google Scholar]
- 22. El-Neweshy MS, El-Maddawy ZK, El-Sayed YS. Therapeutic effects of date palm (Phoenix dactylifera L.) pollen extract on cadmium-induced testicular toxicity. Andrologia. 2013:45(6):369–378. [DOI] [PubMed] [Google Scholar]
- 23. Otify AM, Hammam AMM, Aly Farag M. Phoenix dactylifera L. date tree pollen fertility effects on female rats in relation to its UPLC-MS profile via a biochemometric approach. Steroids. 2021:173:108888. [DOI] [PubMed] [Google Scholar]
- 24. Elkerm Y, Tawashi R. Date palm pollen as a preventative intervention in radiation- and chemotherapy-induced oral Mucositis. Integr Cancer Ther. 2014:13(6):468–472. [DOI] [PubMed] [Google Scholar]
- 25. Shahriarinour M, Divsar F. Release kinetics and antibacterial property of curcumin-loaded date palm (Phoenix dactylifera L.) pollen. Arab J Sci Eng. 2023:48(6):7263–7272. [Google Scholar]
- 26. Kalpana VN, Devi Rajeswari V. A review on green synthesis, biomedical applications, and toxicity studies of ZnO NPs. Bioinorg Chem Appl. 2018:2018:1–12. 10.1155/2018/3569758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. M. el-Shorbagy H, M. Eissa S, Sabet S, . el-Ghor A, A. Apoptosis and oxidative stress as relevant mechanisms of antitumor activity and genotoxicity of ZnO-NPs alone and in combination with N-acetyl cysteine in tumor-bearing mice. Int J Nanomedicine. 2019:14:3911–3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oberdörster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, Ausman K, Carter J, Karn B, Kreyling W, Lai D, et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Part Fibre Toxicol. 2005:2(1):1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hackenberg S, Scherzed A, Harnisch W, Froelich K, Ginzkey C, Koehler C, Hagen R, Kleinsasser N. Antitumor activity of photo-stimulated zinc oxide nanoparticles combined with paclitaxel or cisplatin in HNSCC cell lines. J Photochem Photobiol B Biol. 2012:114:87–93. [DOI] [PubMed] [Google Scholar]
- 30. Baek M, Chung HE, Yu J, Lee JA, Kim TH, Oh JM, Lee WJ, Paek SM, Lee JK, Jeong J, et al. Pharmacokinetics, tissue distribution, and excretion of zinc oxide nanoparticles. Int J Nanomedicine. 2012:7:3081–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sharma V, Singh P, Pandey AK, Dhawan A. Induction of oxidative stress, DNA damage and apoptosis in mouse liver after sub-acute oral exposure to zinc oxide nanoparticles. Mutat Res. 2012:745(1–2):84–91. [DOI] [PubMed] [Google Scholar]
- 32. Lin YF, Chiu IJ, Cheng FY, Lee YH, Wang YJ, Hsu YH, Chiu HW. The role of hypoxia-inducible factor-1α in zinc oxide nanoparticle-induced nephrotoxicity in vitro and in vivo. Part Fibre Toxicol. 2016:13(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barguilla I, Barszczewska G, Annangi B, Domenech J, Velázquez A, Marcos R, Hernández A. MTH1 is involved in the toxic and carcinogenic long-term effects induced by zinc oxide and cobalt nanoparticles. Arch Toxicol. 2020:94(6):1973–1984. [DOI] [PubMed] [Google Scholar]
- 34. Han Z, Yan Q, Ge W, Liu ZG, Gurunathan S, De Felici M, Shen W, Zhang XF. Cytotoxic effects of ZnO nanoparticles on mouse testicular cells. Int J Nanomedicine. 2016:11:5187–5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hegazy A, A, Ahmed M, M, Shehata MA, Abdelfattah MM. Changes in rats’ liver structure induced by zinc oxide nanoparticles and the possible protective role of vitamin E. Int J Hum Anat. 2018:1(3):1–16. [Google Scholar]
- 36. Hamza RZ, al-Eisa RA, el-Shenawy NS. Possible ameliorative effects of the Royal Jelly on hepatotoxicity and oxidative stress induced by molybdenum nanoparticles and/or cadmium chloride in male rats. Biology (Basel). 2022:11(3):450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Abdelhalim MAK, Moussa SAA, Qaid HAY, al-Ayed M. Potential effects of different natural antioxidants on inflammatory damage and oxidative-mediated hepatotoxicity induced by gold nanoparticles. Int J Nanomedicine. 2018:13:7931–7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Abdelhalim MAK, Qaid HAY, Al-Mohy YH, Ghannam MM. The protective roles of vitamin E and α-Lipoic acid against nephrotoxicity, lipid peroxidation, and inflammatory damage induced by gold nanoparticles. Int J Nanomedicine. 2020:15:729–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ben, Slama I. Sub-acute oral toxicity of zinc oxide nanoparticles in male rats. J Nanomed Nanotechnol. 2015:6(3):1000284. 10.4172/2157-7439.1000284. [DOI] [Google Scholar]
- 40. Bin-Jumah MN, AL-Huqail AA, Abdelnaeim N, Kamel M, Fouda MMA, Abulmeaty MMA, Saadeldin IM, Abdel-Daim MM. Potential protective effects of Spirulina platensis on liver, kidney, and brain acrylamide toxicity in rats. Environ Sci Pollut Res. 2021:28(21):26653–26663. [DOI] [PubMed] [Google Scholar]
- 41. El-Tantawy WH. Antioxidant effects of spirulina supplement against lead acetate-induced hepatic injury in rats. J Tradit Complement Med. 2016:6(4):327–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Al-Asmari AK, Al-Said MS, Abbasmanthiri R, Al-Buraidi A, Ibrahim KE, Rafatullah S. Impact of date palm pollen (Phoenix dactylifera) treatment on paracetamol-induced hepatorenal toxicity in rats. Clin Phytoscience. 2020:6(1):1–12. [Google Scholar]
- 43. Deore MS, S K, Naqvi S, Kumar A, Flora SJS. Alpha-Lipoic acid protects co-exposure to lead and zinc oxide nanoparticles induced neuro, immuno and male reproductive toxicity in rats. Front Pharmacol. 2021:12:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moatamed ER, Hussein AA, El-desoky MM, Khayat ZEL. Comparative study of zinc oxide nanoparticles and its bulk form on liver function of Wistar rat. Toxicol Ind Health. 2019:35(10):627–637. [DOI] [PubMed] [Google Scholar]
- 45. Elshopakey GE, Elazab ST. Cinnamon aqueous extract attenuates diclofenac sodium and Oxytetracycline mediated Hepato-renal toxicity and modulates oxidative stress, cell apoptosis, and inflammation in male albino rats. Vet Sci. 2021:8(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gella FJ, Olivella T, Pastor MC, Arenas J, Moreno R, Durban R, Gomez JA. A simple procedure for the routine determination of aspartate aminotransferase and alanine aminotransferase with pyridoxal phosphate. Clin Chim Acta. 1985:153(3):241–247. [DOI] [PubMed] [Google Scholar]
- 47. Rosalki SB, Foo AY, Burlina A, Prellwitz W, Stieber P, Neumeier D, Klein G, Poppe WA, Bodenmuller H. Multicenter evaluation of Iso-ALP test kit for measurement of bone alkaline phosphatase activity in serum and plasma. Clin Chem. 1993:39(4):648–652. [PubMed] [Google Scholar]
- 48. Keiding R, Hörder M, Denmark WG, Pitkänen E, Tenhunen R, Strömme JH, Theodorsen L, Waldenström J, Tryding N, Westlund L. Recommended methods for the determination of four enzymes in blood. Scand J Clin Lab Invest. 1974:33(4):291–306. [DOI] [PubMed] [Google Scholar]
- 49. Webster D, Bignell AHC, Attwood EC. An assessment of the suitability of bromocresol green for the determination of serum albumin. Clin Chim Acta. 1974:53(1):101–108. [DOI] [PubMed] [Google Scholar]
- 50. Young DS. Effects of drugs on clinical laboratory tests. Ann Clin Biochem. 1997:34(6):579–581. [DOI] [PubMed] [Google Scholar]
- 51. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979:95(2):351–358. [DOI] [PubMed] [Google Scholar]
- 52. Kei S. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978:90(1):37–43. [DOI] [PubMed] [Google Scholar]
- 53. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967:70(1):158–169. [PubMed] [Google Scholar]
- 54. Aebi H. [13] Catalase in vitro. Methods Enzymol. 1984:105:121–126. [DOI] [PubMed] [Google Scholar]
- 55. Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, Khorasani S, Mozafari MR. Impact of particle size and Polydispersity index on the clinical applications of Lipidic Nanocarrier systems. Pharmaceutics. 2018:10(2):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Khan M, Naqvi AH, Ahmad M. Comparative study of the cytotoxic and genotoxic potentials of zinc oxide and titanium dioxide nanoparticles. Toxicol Rep. 2015:2:765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mahalakshmi S, Hema N, Vijaya PP. In vitro biocompatibility and antimicrobial activities of zinc oxide nanoparticles (ZnO NPs) prepared by chemical and green synthetic route—a comparative study. Bionanoscience. 2020:10(1):112–121. [Google Scholar]
- 58. Vijayalakshmi U, Chellappa M, Anjaneyulu U, Manivasagam G, Sethu S. Influence of coating parameter and sintering atmosphere on the corrosion resistance behavior of Electrophoretically deposited composite coatings. Mater Manuf Process. 2016:31(1):95–106. [Google Scholar]
- 59. Saman S, Moradhaseli S, Shokouhian A, Ghorbani M. Histopathological effects of ZnO nanoparticles on liver and heart tissues in Wistar rats. Adv Biores. 2013:4:83–88. [Google Scholar]
- 60. Sudhakaran S, Athira SS, Mohanan PV. Zinc oxide nanoparticle induced neurotoxic potential upon interaction with primary astrocytes. Neurotoxicology. 2019:73:213–227. [DOI] [PubMed] [Google Scholar]
- 61. Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006:311(5761):622–627. [DOI] [PubMed] [Google Scholar]
- 62. Hillyer JF, Albrecht RM. Gastrointestinal persorption and tissue distribution of differently sized colloidal gold nanoparticles. J Pharm Sci. 2001:90(12):1927–1936. [DOI] [PubMed] [Google Scholar]
- 63. Srivastav AK, Kumar M, Ansari NG, Jain AK, Shankar J, Arjaria N, Jagdale P, Singh D. A comprehensive toxicity study of zinc oxide nanoparticles versus their bulk in Wistar rats. Hum Exp Toxicol. 2016:35(12):1286–1304. [DOI] [PubMed] [Google Scholar]
- 64. Hong JS, Park MK, Kim MS, Lim JH, Park GJ, Maeng EH, Shin JH, Kim MK, Jeong J, Park JA, et al. Prenatal development toxicity study of zinc oxide nanoparticles in rats. Int J Nanomedicine. 2014:9:159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Abbasalipourkabir R, Moradi H, Zarei S, Asadi S, Salehzadeh A, Ghafourikhosroshahi A, Mortazavi M, Ziamajidi N. Toxicity of zinc oxide nanoparticles on adult male Wistar rats. Food Chem Toxicol. 2015:84:154–160. [DOI] [PubMed] [Google Scholar]
- 66. Marin P, Israël M, Glowinski J, Prémont J. Routes of zinc entry in mouse cortical neurons: role in zinc‐induced neurotoxicity. Eur J Neurosci. 2000:12(1):8–18. [DOI] [PubMed] [Google Scholar]
- 67. Oettl K, Stauber RE. Physiological and pathological changes in the redox state of human serum albumin critically influence its binding properties. Br J Pharmacol. 2007:151(5):580–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shaban EE, Ibrahim KS, El-Sayed EM, Abd El-Aziz ME, Nasr SM, Desouky HM, Elbakry HF. Evaluation of acute oral toxicity of zinc oxide nanoparticles in rats. Egypt J Chem. 2021:64:4591–4600. [Google Scholar]
- 69. Xiao L, Liu C, Chen X, Yang Z. Zinc oxide nanoparticles induce renal toxicity through reactive oxygen species. Food Chem Toxicol. 2016:90:76–83. [DOI] [PubMed] [Google Scholar]
- 70. Sharma V, Anderson D, Dhawan A. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2). Apoptosis. 2012:17(8):852–870. [DOI] [PubMed] [Google Scholar]
- 71. Attia H, Nounou H, Shalaby M. Zinc oxide nanoparticles induced oxidative DNA damage, inflammation and apoptosis in Rat's brain after oral exposure. Toxics. 2018:6(2):29, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kvietys PR, Granger DN. Role of reactive oxygen and nitrogen species in the vascular responses to inflammation. Free Radic Biol Med. 2012:52(3):556–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Park EJ, Park K. Oxidative stress and pro-inflammatory responses induced by silica nanoparticles in vivo and in vitro. Toxicol Lett. 2009:184(1):18–25. [DOI] [PubMed] [Google Scholar]
- 74. Fujihara J, Tongu M, Hashimoto H, Fujita Y, Nishimoto N, Yasuda T, Takeshita H. Pro-inflammatory responses and oxidative stress induced by ZnO nanoparticles in vivo following intravenous injection. Eur Rev Med Pharmacol Sci. 2015:19(24):4920–4926. [PubMed] [Google Scholar]
- 75. Vandebriel RJ, De Jong WH. A review of mammalian toxicity of ZnO nanoparticles. Nanotechnol Sci Appl. 2012:5:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Schrand AM, Rahman MF, Hussain SM, Schlager JJ, Smith DA, Syed AF. Metal‐based nanoparticles and their toxicity assessment. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010:2(5):544–568. [DOI] [PubMed] [Google Scholar]
- 77. Ma L, Zhao J, Wang J, Liu J, Duan Y, Liu H, Li N, Yan J, Ruan J, Wang H, et al. The acute liver injury in mice caused by Nano-Anatase TiO2. Nanoscale Res Lett. 2009:4(11):1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Almansour MI, Alferah MA, Shraideh ZA, Jarrar BM. Zinc oxide nanoparticles hepatotoxicity: histological and histochemical study. Environ Toxicol Pharmacol. 2017:51:124–130. [DOI] [PubMed] [Google Scholar]
- 79. Esmaeillou M, Moharamnejad M, Hsankhani R, Tehrani AA, Maadi H. Toxicity of ZnO nanoparticles in healthy adult mice. Environ Toxicol Pharmacol. 2013:35(1):67–71. [DOI] [PubMed] [Google Scholar]
- 80. Najafzadeh H, Ghoreishi SM, Mohammadian B, Rahimi E, Afzalzadeh MR, Kazemivarnamkhasti M, Ganjealidarani H. Serum biochemical and histopathological changes in liver and kidney in lambs after zinc oxide nanoparticles administration. Vet World. 2013:6(8):534–537. [Google Scholar]
- 81. Abdel-Daim MM, Abuzead SMM, Halawa SM. Protective role of Spirulina platensis against acute deltamethrin-induced toxicity in rats. PLoS One. 2013:8(9):e72991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pérez-Juárez A, Aguilar-Faisal JL, Posadas-Mondragón A, Santiago-Cruz JA, Barrientos-Alvarado C, Mojica-Villegas MA, Chamorro-Cevallos GA, Morales-González JA. Effect of spirulina (formerly Arthrospira) maxima against ethanol-induced damage in rat liver. Appl Sci. 2022:12(17):8626. [Google Scholar]
- 83. Minic S, Stanic-Vucinic D, Radomirovic M. 1–9.
- 84. Radibratovic M, Minic S, Stanic-Vucinic D, Nikolic M, Milcic M, Cirkovic Velickovic T. Stabilization of human serum albumin by the binding of phycocyanobilin, a bioactive chromophore of blue-green alga spirulina: molecular dynamics and experimental study. PLoS One. 11(12):e0167973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Jakobek L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015:175:556–567. [DOI] [PubMed] [Google Scholar]
- 86. El Bialy BE, El-Boraey NG, Hamouda RA, Abdel-Daim MM. Comparative protective effects of spirulina and spirulina supplemented with Thiamineagainst sub-acute carbon tetrachloride toxicity in rats. Biomed Pharmacol J. 2019:12(2):511–526. [Google Scholar]
- 87. Jang MH, Piao XL, Kim JM, Kwon SW, Park JH. Inhibition of cholinesterase and amyloid-β aggregation by resveratrol oligomers from vitis amurensis. Phytother Res. 2008:22(4):544–549. [DOI] [PubMed] [Google Scholar]
- 88. Ali EAI, Barakat BM, Hassan R. Antioxidant and Angiostatic effect of Spirulina platensis suspension in complete Freund’s adjuvant-induced arthritis in rats. PLoS One. 2015:10(4):e0121523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Shokri H, Khosravi A, Taghavi M. Efficacy of Spirulina platensis on immune functions in cancer mice with systemic candidiasis. J Mycol Res. 2014:1(1):7–13. [Google Scholar]
- 90. Miranda MS, Cintra RG, Barros SBM, Mancini-Filho J. Antioxidant activity of the microalga Spirulina maxima. Braz J Med Biol Res. 1998:31(8):1075–1079. [DOI] [PubMed] [Google Scholar]
- 91. Bhat VB, Madyastha KM. C-Phycocyanin: A potent Peroxyl radical scavenger in vivo and in vitro. Biochem Biophys Res Commun. 2000:275(1):20–25. [DOI] [PubMed] [Google Scholar]
- 92. Tahvilzadeh M, Hajimahmoodi M, Rahimi R. The role of date palm (phoenix dactyliferaL) pollen in fertility. J Evid Based Complementary Altern Med. 2016:21(4):320–324. [DOI] [PubMed] [Google Scholar]
- 93. Xie W, Chen C, Jiang Z, Wang J, Melzig MF, Zhang X. Apocynum venetumAttenuates acetaminophen-induced liver injury in mice. Am J Chin Med. 2015:43(3):457–476. [DOI] [PubMed] [Google Scholar]
- 94. Aseervatham GSB, Sivasudha T, Sasikumar JM, Christabel PH, Jeyadevi R, Ananth DA. Antioxidant and hepatoprotective potential of Pouteria campechiana on acetaminophen-induced hepatic toxicity in rats. J Physiol Biochem. 2014:70(1):1–14. [DOI] [PubMed] [Google Scholar]
- 95. Fatani A, Baothman O, Shash L, Abuaraki H, Zeyadi M, Hosawi S, Altayb H, Abo-Golayel M. Hepatoprotective effect of date palm fruit extract against doxorubicin intoxication in Wistar rats: in vivo and in silico studies. Asian Pac J Trop Biomed. 2022:12(8):357–366. [Google Scholar]
- 96. Abed El Azim MHM. Identification phenolic and biological activities of Methanolic extract of date palm pollen (Phoenix dactylifera). J Microb Biochem Technol. 2015:07(01):47–50. [Google Scholar]
- 97. Kandemir FM, Ozkaraca M, Yildirim BA, Hanedan B, Kirbas A, Kilic K, Aktas E, Benzer F. Rutin attenuates gentamicin-induced renal damage by reducing oxidative stress, inflammation, apoptosis, and autophagy in rats. Ren Fail. 2015:37(3):518–525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All date related to this article are included in the maintext.



