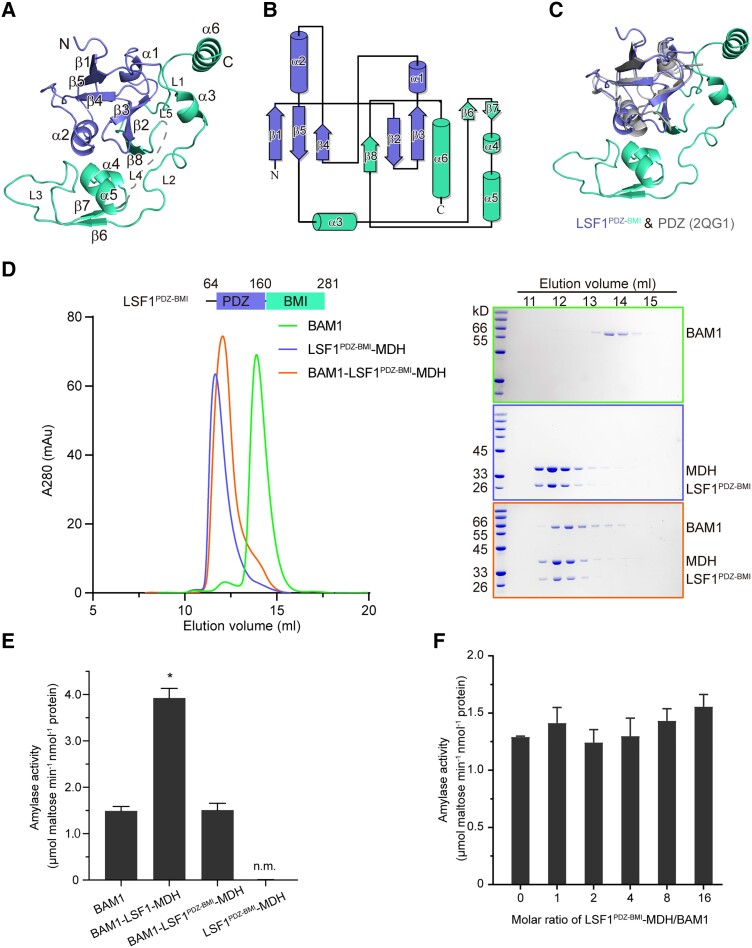
Figure 2.
The LSF1 N terminus is sufficient for ternary complex assembly. A) Structure of the LSF1 N terminus. Secondary structural elements are labeled. The PDZ and BMI domains are colored in slate and green-cyan, respectively. B) Topological model of the LSF1 N terminus. The number of secondary structural elements are labeled. C) Structural superposition of the LSF1 N terminus with the canonical PDZ domain. The structure of 2QG1 is shown in gray. D) The N-terminal PDZ-BMI domain of LSF1 is sufficient to mediate ternary complex formation. Left, a representative gel filtration chromatography; right, SDS-PAGE corresponding to the chromatography. E) Starch hydrolytic activity examined for the BAM1–LSF1PDZ-BMI–MDH complex. n.m., no measured activity. Two-tailed Student's t-tests are used for statistical analysis (*P < 0.05). Error bar, Sd. F) Titration assay of the starch-degradation activity of BAM1 upon the addition of the binary LSF1PDZ-BMI–MDH complex with increased molar ratios. LSF1PDZ-BMI–MDH is unable to promote the starch hydrolytic activity of BAM1. Two-tailed Student's t-tests are used for statistical analysis (*P < 0.05). Error bar, Sd.