Abstract
Carotenoids have been recognized for their potential health benefits due to their antioxidant properties. There is limited research on the association between metabolic dysfunction-associated fatty liver disease (MAFLD) and carotenoids. This study aimed to investigate the effect of carotenoid intake on the risk of MAFLD. We retrospectively analyzed 2722 adults aged ≥ 18 from the National Health and Nutrition Examination Survey 2017-2018. Hepatic steatosis was identified by elastography, and carotenoid consumption was evaluated through two 24-hour dietary recall interviews. Weighted logistic regression models, subgroup analyses, and restricted cubic splines were used for analyses. The weighted prevalence of MAFLD was 51.90%. Weighted logistic regression analysis demonstrated that intake of β-carotene, lutein/zeaxanthin, and lycopene was associated with a lower risk of MAFLD after adjusting for various covariates. Compared to the lowest tertile, a significant inverse correlation was observed between the highest total lycopene intake and MAFLD among females in the gender subgroup analysis. Restricted cubic spline regression analysis revealed a U-shaped association between lycopene consumption and MAFLD risk (P < .001), with an inflection point of approximately 9.48 mg/day. Moreover, the nonlinear relationship was particularly significant in females and absent in males. In summary, increased β-carotene, lutein/zeaxanthin, and lycopene consumption was associated with a decreased risk of MAFLD. The relationship between total lycopene intake and MAFLD was nonlinear, primarily in females. These findings have significant implications for the potential prevention and management of MAFLD.
Keywords: carotenoids, MAFLD, lycopene, hepatic steatosis, NHANES
1. Introduction
Metabolic dysfunction-associated fatty liver disease (MAFLD), a newly proposed definition to replace nonalcoholic fatty liver disease (NAFLD), serves as a “positive criteria” independent of excessive alcohol consumption or other concomitant liver diseases and is correlated with the patient’s metabolic risk profile.[1] As a multi-systemic disease involving obesity, diabetes, and metabolic syndrome, MAFLD affects approximately 39% of the population, and the risk of advanced liver fibrosis in MAFLD patients is as high as 7.4%, making it an increasingly prominent cause of chronic liver failure requiring liver transplantation worldwide.[2,3] People living with MAFLD also have higher all-cause, cardiovascular-related, and other-cause mortality based on the findings of the Third National Health and Nutrition Examination Survey (NHANES III), while currently having no approved specific pharmaceutical treatments.[4] Therefore, identifying modifiable risk factors and implementing effective interventions are crucial because of the growing economic and health burden of MAFLD.
Carotenoids, primarily diet-derived micronutrients, have potential health benefits in reducing the risk of certain cancers, cardiovascular diseases, and macular degeneration.[5–7] Individuals with high-level carotenoids also experience a lower incidence of chronic obstructive pulmonary disease, all-cause dementia, and chronic kidney disease among American adults.[8–10] According to research conducted through the NHANES 2003 to 2014, the risk of NAFLD decreased with increasing intake of carotenoids, including α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin, and lycopene.[11] As natural antioxidants, the consumption of β-carotene and lutein/zeaxanthin was found to be inversely correlated with liver steatosis.[12] In addition, lycopene has been shown to ameliorate lipid accumulation and hepatocyte steatosis in both vivo and vitro models of NAFLD, which may be related to the “multiple-hit” hypothesis embracing diverse processes, such as insulin resistance, lipotoxicity, inflammation, and cytokines imbalance, among others.[13] However, limited research exists on the association between carotenoids and MAFLD.
The objective of this study was to investigate whether carotenoid consumption could serve as a preventive factor against MAFLD, which highlights metabolic dysfunction as the core of the disease.[14] For this purpose, we mainly conducted logistic regression analysis and restricted cubic spline analysis to ascertain and visualize the association between carotenoid intake and the risk of MAFLD among American adults by utilizing the NHANES database, thereby proposing novel insights into the prevention and management of MAFLD.
2. Materials and Methods
2.1. Study design and participants
The NHANES program is carried out by the National Centre for Health Statistics to monitor the nutrition and health status of American adults and children by examining a nationwide representational sample of around 5000 individuals annually.[15] This cross-sectional study extracted data from the NHANES 2017 to 2018 cycle, which was the first to use FibroScan® for ultrasound and vibration-controlled transient elastography (VCTE) to obtain liver stiffness measurements and controlled attenuation parameters (CAPs) as indicators for evaluating liver fibrosis and hepatic steatosis, respectively.
Elastography measurements were performed at the NHANES mobile examination center (MEC) by a trained healthcare technician using the FibroScan® model 502 V2 Touch, which was equipped with either a medium or extra-large wand (probe). The validity of the VCTE examination requires 3 essential requirements: a minimum fasting duration of 3 hours, at least 10 complete stiffness (E) measurements, and a liver stiffness interquartile range/median E < 30%. Elastography is a noninvasive diagnostic technology that has been shown to reflect liver fibrosis (sensitivity, 93.7%; specificity, 91.1%) and hepatic steatosis (with an AUROC of 0.96).[16–18] Additional details on the protocols for quality control and assurance during the VCTE procedures can be found in publicly accessible resources.[19]
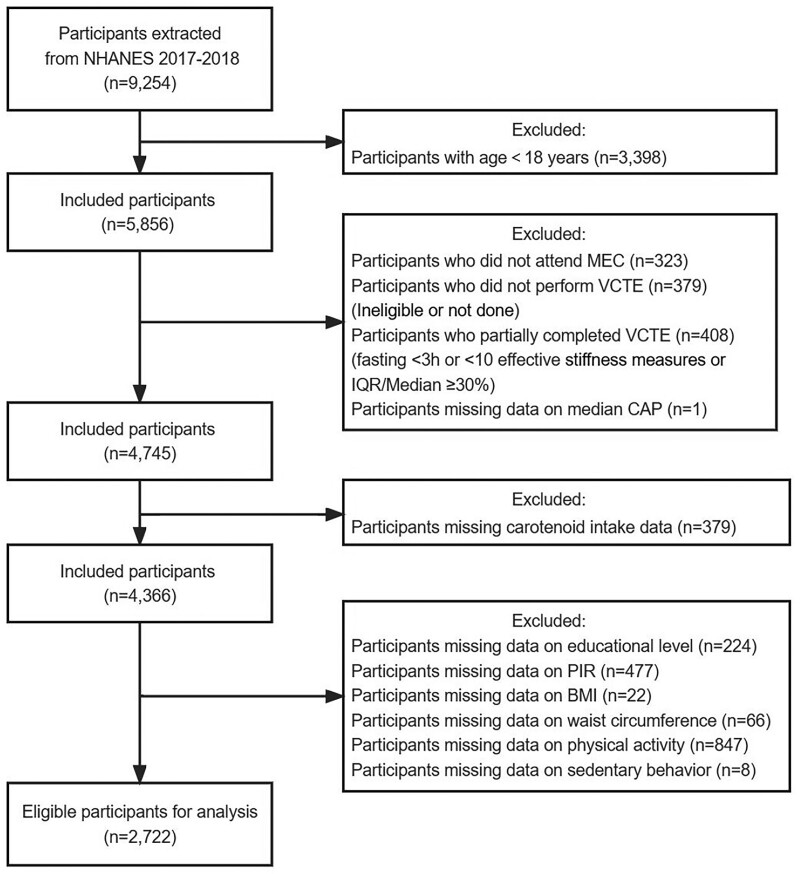
Of the 9254 participants in NHANES 2017 to 2018, 5856 aged ≥ 18 years initially enrolled were required to attend the MEC, but 323 only underwent interviews, 379 did not perform the exam (ineligible or not done), 408 partially completed (fasting < 3 hours or < 10 effective E measures or interquartile range/Median ≥ 30%) and 1 lacked the median CAP value. Furthermore, 379 individuals missing data on dietary carotenoid intake were excluded. After further excluding individuals with insufficient data on other covariates, 2722 participants with complete information about sociodemographic data, physiological measurements, carotenoid intake, and comorbidities (hypertension, diabetes mellitus, and metabolic syndrome) remained in the final analysis, comprising 1476 individuals with MAFLD and 1246 without (Fig. 1). All participants provided informed consent for this study, which was authorized by the National Centre for Health Statistics Research Ethics Review Board (Protocol number: 2018-01) before enrollment.
Figure 1.
Flowchart of the sample selection from NHANES 2017–2018. BMI = body mass index, CAP = controlled attenuation parameter, IQR = interquartile range, NHANES = National Health and Nutrition Examination Survey, MEC = mobile examination center, VCTE = vibration-controlled transient elastography, PIR = poverty-income ratio.
2.2. Definition of MAFLD
Under the new definition proposed by the 2020 international expert consensus statement,[1] the diagnostic criteria for MAFLD require the presence of hepatic steatosis detected through imaging techniques, blood biomarkers, or liver biopsy, in conjunction with at least one of the following 3 criteria: overweight/obesity, type 2 diabetes mellitus, or metabolic dysregulation. This study utilized an optimal CAP cutoff of 248 dB/m to identify hepatic steatosis, achieving a sensitivity of 68.8%, specificity of 82.2%, and a maximum Youden index.[20] MAFLD status was defined as non-MAFLD or MAFLD based on the median CAP by VCTE measurements.
2.3. Carotenoid intake assessment
All NHANES participants were available to provide detailed information on their dietary intake and supplement usage to assess nutrient, energy, and other food constituent intakes during two 24-hour dietary recall interviews. The initial dietary recall interview was conducted face-to-face at the MEC, whereas the subsequent one was obtained via telephone 3 to 10 days later. To enhance the accuracy of the estimates, the consumption of α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin, and lycopene from the diet were averaged across 2 recall periods (the value was utilized instead of the average if only the initial recall were available). However, only lutein/zeaxanthin and lycopene intake from supplements were collected in this specific cycle, and these supplement intakes were also averaged over 2 recalls if available. Consequently, total lutein/zeaxanthin and total lycopene intake were computed by adding both dietary and supplementary sources.
2.4. Other covariates
Data on demographics, lifestyle, and health status were obtained through the Computer Assisted Personal Interview system during the household interviews. Participants were classified into different groups, including Mexican American, non-Hispanic White, non-Hispanic Black, non-Hispanic Asian, and other/multiracial, based on the reported race and Hispanic origin information. Education level was categorized into 3 groups: less than high school, high school or equivalent, and college degree or above. The poverty-income ratio (PIR) was categorized as low ( < 1.30), middle (1.30 ≤ PIR < 3.50), and high (≥3.50).[21] We divided physical activity into low ( < 600), moderate (600– < 8000), and high (≥8000) levels by the total weekly minutes metabolic equivalent (MET minute/week) of participants vigorous, moderate work/recreational activity, as well as walking or bicycling.[22] Smoking status was determined by the “Smoking-Cigarette Use” questionnaire into never smokers (having smoked < 100 cigarettes in their lifetime), former smokers (having smoked at least 100 cigarettes in their lifetime but currently quit), and current smokers (having smoked at least 100 cigarettes in their lifetime and still smoking at present). Hypertension was defined by the previous diagnosis, taking prescription medication for hypertension, or an average blood pressure ≥ 140/90 mm Hg.[23] Diabetes mellitus was diagnosed based on either (a) Doctor told you have diabetes or (b) Fasting plasma glucose (FPG) ≥ 7.0 mmol/L or (c) 2-hour PG ≥ 11.1 mmol/L during oral glucose tolerance test or (d) Glycohemoglobin (HbA1c) ≥ 6.5% or (e) Random plasma glucose ≥ 11.1 mmol/L or (f) Use of diabetes medication or insulin.[24] The diagnosis criteria for metabolic syndrome in clinical practice maintained the minor modified version of the Adult Treatment Panel III definition, which has been widely accepted in the United States and other countries.[25,26]
Blood samples and physiological examinations were collected following standardized procedures during the participant’s MEC visit. The whole blood and serum specimens underwent processing, storage, and shipping to either the Advanced Research Diagnostics Laboratory at the University of Minnesota or the University of Missouri-Columbia for analysis. In this study, we extracted various laboratory indicators, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (γ-GT), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), fasting insulin (FINS), FPG, HbA1c, and high-sensitivity C-reactive protein (Hs-CRP). Further information regarding questionnaire instruments, exam procedure manuals, laboratory methods, and guidelines for dietary recall interviews is accessible on the NHANES website.
2.5. Statistical analysis
Following the recommendations provided in the NHANES analytic notes, the dietary day 1 sample weight (WTDRD1) was applied as an appropriate sample weight to this analysis, considering the multi-stage, unequal probability selection design of the NHANES survey. Based on MAFLD status (non-MAFLD vs MAFLD), we summarized the participants’ baseline characteristics. Weighted means with 95% confidence intervals (CIs) were used to present continuous variables, whereas weighted percentages (95% CIs) were used for categorical variables. Weighted linear regression and weighted Chi-square tests were used for continuous and categorical variables to compare the differences between the 2 MAFLD phenotypes in baseline characteristics. The analyses conducted did not use imputation methods for any variable.
Weighted logistic regression models were conducted to explore the association between carotenoid intake and MAFLD. To verify the association and investigate the potential nonlinear relationship, carotenoids, which were continuous, were discretized into tertiles, and the P values for trends were computed. Three models were established in this study and were stratified by gender. No adjustments were made to the basic model for any possible confounding variable. Model 1 was adjusted for age, gender, race/ethnicity, educational level, and PIR. In Model 2, additional adjustments were made for body mass index, waist circumference, physical activity, sedentary behavior, smoking status, hypertension, diabetes mellitus, metabolic syndrome, ALT, AST, γ-GT, TG, LDL-C, FINS, FPG, HbA1c, Hs-CRP, and total calories. In sensitivity analyses, we recalculated carotenoid intake by selecting participants with both days of dietary carotenoid intake and using dietary 2-day sample weight (WTDR2D), and then analyzed the association of new carotenoid intake with MAFLD. Furthermore, multiplicative interaction tests were conducted to ascertain whether there was an interaction between gender and carotenoid intake. Restricted cubic splines incorporating 3 knots positioned at the 10th, 50th, and 90th percentiles were employed to flexibly model the association between MAFLD and carotenoid intake.
The R software (https://www.r-project.org/, accessed on March 16 2023, 4.2.3 version, The R Foundation for Statistical Computing, Vienna, Austria) was used for all statistical analyses.
3. Results
3.1. Baseline characteristics of participants with and without MAFLD
Table 1 displays the baseline characteristics of the study participants categorized by MAFLD status. The weighted prevalence of MAFLD in the entire population of the United States was 51.90%. Of the 2722 participants ultimately enrolled in the study, the overall weighted mean age was 46.31 years, 47.80% were female, and the majority of subjects were non-Hispanic White (weighted percentage: 64.00%). Compared to participants without MAFLD, those with MAFLD were more likely to be older, male, obese, have a larger waist circumference, and have elevated levels of ALT, γ-GT, TG, LDL-C, FINS, FPG, H1Abc, Hs-CRP, and median CAP, as well as a higher incidence of hypertension, metabolic syndrome, and diabetes mellitus. Significant differences in race/ethnicity, educational attainment, physical activity, and smoking status were also observed between the 2 groups, while no significant differences were observed in AST, PIR, and sedentary behavior. The MAFLD group had lower consumption of dietary α-carotene, β-carotene, and total lutein/zeaxanthin than the non-MAFLD group. In contrast, the differences in total calories, dietary β-cryptoxanthin, and total lycopene intake were not statistically significant.
Table 1.
Baseline characteristics of participants.
| Characteristics | Overall(N = 2722)* | Non-MAFLD (N = 1246)* | MAFLD (N = 1476)* | P value† |
|---|---|---|---|---|
| Age (yr) | 46.31 (44.85, 47.77) | 42.53 (41.07, 43.99) | 49.82 (48.06, 51.57) | <.001 |
| Gender, % | <.001 | |||
| Male | 52.20 (46.55, 57.86) | 46.89 (43.00, 50.79) | 57.12 (53.50, 60.75) | |
| Female | 47.80 (43.99, 51.60) | 53.11 (49.21, 57.00) | 42.88 (39.25, 46.50) | |
| Race/ethnicity, % | <.001 | |||
| Mexican American | 8.88 (5.63, 12.14) | 5.97 (3.72, 8.23) | 11.58 (7.56, 15.59) | |
| Non-Hispanic White | 64.00 (54.60, 73.39) | 66.56 (60.08, 73.05) | 61.62 (54.23, 69.00) | |
| Non-Hispanic Black | 10.21 (7.69, 12.74) | 11.61 (8.51, 14.71) | 8.92 (5.43, 12.41) | |
| Non-Hispanic Asian | 5.93 (4.37, 7.49) | 6.08 (4.05, 8.12) | 5.78 (3.85, 7.71) | |
| Other/multiracial | 10.98 (8.60, 13.37) | 9.77 (7.05, 12.49) | 12.11 (9.44, 14.77) | |
| Educational level, % | .005 | |||
| Less than high school | 8.77 (7.49, 10.05) | 7.97 (6.18, 9.75) | 9.51 (7.88, 11.13) | |
| High school or equivalent | 27.58 (22.76, 32.41) | 25.29 (18.91, 31.67) | 29.71 (26.12, 33.30) | |
| College or above | 63.65 (57.14, 70.16) | 66.74 (59.98, 73.51) | 60.78 (56.97, 64.60) | |
| Poverty-income ratio, % | .260 | |||
| Low (<1.30) | 19.15 (17.00, 21.30) | 19.40 (16.72, 22.09) | 18.92 (16.48, 21.35) | |
| Middle (1.30 to < 3.50) | 35.43 (29.52, 41.34) | 33.88 (28.10, 39.67) | 36.86 (30.74, 42.99) | |
| High (≥3.50) | 45.42 (39.91, 50.93) | 46.71 (41.29, 52.14) | 44.22 (38.13, 50.31) | |
| Body mass index (kg/m2), % | <.001 | |||
| Underweight or Normal (<25) | 28.40 (24.31, 32.49) | 52.94 (48.60, 57.27) | 5.67 (4.26, 7.08) | |
| Overweight (25 to < 30) | 30.71 (26.98, 34.44) | 29.32 (24.97, 33.67) | 32.01 (27.45, 36.56) | |
| Obese (30 or greater) | 40.89 (35.59, 46.18) | 17.75 (13.05, 22.45) | 62.33 (57.64, 67.01) | |
| Waist circumference (cm) | 99.54 (98.20, 100.89) | 89.34 (88.11, 90.56) | 109.00 (107.58, 110.42) | <.001 |
| Physical activity (MET min/week), % | <.001 | |||
| Low (<600) | 13.06 (10.94, 15.19) | 9.19 (6.46, 11.92) | 16.65 (13.57, 19.73) | |
| Moderate (600 to < 8000) | 62.27 (57.69, 66.84) | 66.62 (62.52, 70.71) | 58.24 (53.94, 62.53) | |
| High (≥8000) | 24.67 (20.42, 28.92) | 24.19 (20.45, 27.93) | 25.11 (21.63, 28.59) | |
| Sedentary behavior (min/day) | 334.64 (319.08, 350.20) | 331.51 (310.84, 352.17) | 337.55 (318.96, 356.13) | .400 |
| Smoking status, % | <.001 | |||
| Never smoker | 58.72 (54.14, 63.30) | 61.29 (57.49, 65.10) | 56.34 (52.59, 60.08) | |
| Former smoker | 23.77 (20.65, 26.89) | 20.20 (17.33, 23.08) | 27.07 (23.36, 30.79) | |
| Current smoker | 17.51 (13.74, 21.28) | 18.50 (14.45, 22.56) | 16.59 (13.36, 19.82) | |
| Hypertension, % | 35.51 (31.07, 39.96) | 30.04 (26.21, 33.87) | 69.96 (66.13, 73.79) | <.001 |
| Diabetes mellitus, % | 12.33 (10.58, 14.07) | 16.46 (11.19, 21.74) | 83.54 (78.26, 88.81) | <.001 |
| Metabolic syndrome, % | 28.37 (24.48, 32.26) | 15.22 (11.11, 19.33) | 84.78 (80.67, 88.89) | <.001 |
| Alanine aminotransferase (U/L) | 23.96 (22.99, 24.92) | 20.29 (18.20, 22.39) | 27.28 (26.19, 28.36) | <.001 |
| Aspartate aminotransferase (U/L) | 22.87 (22.03, 23.71) | 22.33 (20.27, 24.38) | 23.36 (22.54, 24.19) | .060 |
| Gamma-glutamyl transferase (IU/L) | 29.40 (27.87, 30.93) | 23.41 (21.22, 25.60) | 34.83 (31.64, 38.03) | <.001 |
| Triglyceride (mmol/L) | 1.28 (1.18, 1.38) | 0.95 (0.88, 1.01) | 1.57 (1.40, 1.74) | <.001 |
| LDL-cholesterol (mmol/L) | 2.89 (2.82, 2.97) | 2.81 (2.71, 2.91) | 2.97 (2.80, 3.14) | .002 |
| Fasting insulin (uU/ml) | 12.35 (11.14, 13.55) | 7.78 (6.99, 8.57) | 16.32 (14.53, 18.10) | <.001 |
| Fasting plasma glucose (mmol/L) | 6.05 (5.93, 6.17) | 5.52 (5.45, 5.59) | 6.51 (6.29, 6.73) | <.001 |
| Glycohemoglobin (%) | 5.60 (5.56, 5.64) | 5.35 (5.31, 5.39) | 5.82 (5.75, 5.90) | <.001 |
| Hs-CRP (mg/L) | 3.57 (3.13, 4.02) | 2.35 (1.96, 2.75) | 4.68 (4.14, 5.22) | <.001 |
| Total calories (kcal/d) | 2133.97 (2081.82, 2186.12) | 2100.02 (2025.95, 2174.10) | 2165.42 (2118.93, 2211.91) | .060 |
| Dietary α-carotene (mg/d) | 0.39 (0.33, 0.45) | 0.44 (0.37, 0.52) | 0.34 (0.29, 0.40) | .002 |
| Dietary β-carotene (mg/d) | 2.47 (2.19, 2.76) | 2.86 (2.43, 3.28) | 2.12 (1.89, 2.35) | <.001 |
| Dietary β-cryptoxanthin (mg/d) | 0.09 (0.08, 0.11) | 0.09 (0.08, 0.11) | 0.09 (0.07, 0.12) | .940 |
| Total Lutein/zeaxanthin (mg/d) | 1.90 (1.68, 2.11) | 2.26 (1.85, 2.67) | 1.56 (1.43, 1.69) | <.001 |
| Total Lycopene (mg/d) | 5.05 (4.53, 5.57) | 5.26 (4.62, 5.90) | 4.85 (4.21, 5.49) | .130 |
| CAP (dB/m) | 260.84 (256.65, 265.03) | 209.38 (206.35, 212.41) | 308.53 (305.42, 311.63) | <.001 |
CAP = controlled attenuation parameter, Hs-CRP = high-sensitivity C-reactive, MAFLD = metabolic dysfunction-associated fatty liver diseaseprotein.
Weighted means or weighted percentages with their 95% confidence intervals (CIs) for continuous or categorical variables.
The P values were calculated by weighted linear regression model or weighted Chi-squared test for continuous or categorical variables.
3.2. Association of carotenoid intake with MAFLD
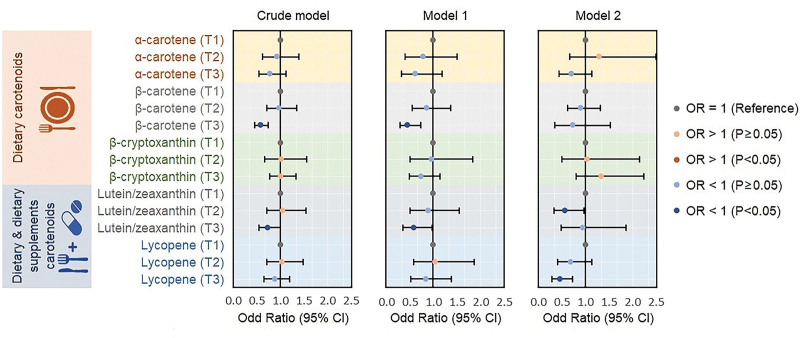
As illustrated in Figure 2, weighted logistic regression analysis demonstrated the association between carotenoid intake and MAFLD. In the unadjusted model, the highest tertile of dietary β-carotene and total lutein/zeaxanthin was inversely correlated with MAFLD compared with the lowest tertile (β-carotene T3 vs T1: OR = 0.579, 95% CI: 0.450–0.744, P < .001; lutein/zeaxanthin T3 vs T1: OR = 0.736, 95% CI: 0.546–0.993, P = .045). Model 1 showed comparable findings after adjusting for sociodemographic characteristics. However, no significant association was observed between dietary β-carotene and MAFLD after full adjustment (Model 2), and the notable negative correlation between the intake of total lutein/zeaxanthin and MAFLD shifted from the highest tertile to the second tertile (lutein/zeaxanthin T2 vs T1: OR = 0.568, 95% CI: 0.333–0.969, P = .039). Furthermore, the risk of MAFLD was obviously reduced in the top tertile of lycopene intake compared to that in the lowest tertile (lycopene T3 vs T1: OR = 0.459, 95% CI: 0.289–0.729, P = .003). In sensitivity analysis, roughly consistent results were observed (see Table S1, Supplemental Digital Content, http://links.lww.com/MD/L81, which illustrates the association between carotenoid intake and MAFLD in sensitivity analysis).
Figure 2.
Weighted logistic regression analysis models showing the association between MAFLD risk and carotenoid intake. Crude model: Unadjusted model. Model 1: Adjusted for age, gender, race/ethnicity, educational level, and poverty-income ratio. Model 2: Additionally adjusted for body mass index, waist circumference, physical activity, sedentary behavior, smoking status, hypertension, diabetes mellitus, metabolic syndrome, ALT, AST, γ-GT, TG, LDL-C, FINS, FPG, HbA1c, Hs-CRP, and total calories. γ-GT = gamma-glutamyl transferase, ALT = alanine aminotransferase, AST = aspartate aminotransferase, FINS = fasting insulin, FPG = fasting plasma glucose, HbA1c = glycohemoglobin, Hs-CRP = high-sensitivity C-reactive protein, LDL-C = low-density lipoprotein cholesterol, MAFLD = metabolic dysfunction-associated fatty liver disease, OR = odd ratio, TG = triglyceride.
3.3. Association between carotenoid intake and MAFLD stratified by gender
Table 2 exhibits the correlation between carotenoid intake and MAFLD in different gender subgroups determined by weighted logistic regression. Significant inverse correlations were found in the crude model between MAFLD and the highest tertile of dietary β-carotene, total lutein/zeaxanthin, and total lycopene consumption in the female group, with all of the above linear trends being statistically significant. Nevertheless, the negative association in females only existed in total lycopene intake after adjusting for sociodemographic characteristics, anthropometric indices, standard biochemical indexes, and comorbidities, and the linear pattern was still significant (P for trend = .027). Regarding the interaction analysis of gender on carotenoid intake, there was an interaction of gender on total lycopene intake in the basic model (P for interaction = .014), which vanished after accounting for confounding factors (P for interaction = .299). Consistent results were also obtained from the sensitivity analysis (see Table S2, Supplemental Digital Content, http://links.lww.com/MD/L82, which demonstrates the association between carotenoid intake and MAFLD in each gender in sensitivity analysis).
Table 2.
Odd ratio estimates for the association between the carotenoid intake and metabolic dysfunction-associated fatty liver disease in each gender.
| Carotenoids | T1 | T2 (OR 95%CI) | P value | T3 (OR 95%CI) | P value | P for trend | P for interaction |
|---|---|---|---|---|---|---|---|
| α-carotene | |||||||
| Unadjusted | .079 | ||||||
| Male | 1.000 | 0.856 (0.522,1.404) | .510 | 0.997 (0.636,1.564) | .989 | .944 | |
| Female | 1.000 | 1.019 (0.613,1.695) | .938 | 0.640 (0.394,1.039) | .068 | .068 | |
| Adjusted* | .810 | ||||||
| Male | 1.000 | 1.031 (0.349, 3.045) | .953 | 0.780 (0.329, 1.851) | .549 | .533 | |
| Female | 1.000 | 1.572 (0.689, 3.589) | .261 | 0.725 (0.402, 1.309) | .264 | .381 | |
| β-carotene | |||||||
| Unadjusted | .028 | ||||||
| Male | 1.000 | 1.094 (0.733,1.631) | .637 | 0.813 (0.560,1.182) | .254 | .261 | |
| Female | 1.000 | 0.857 (0.553,1.327) | .459 | 0.416 (0.283,0.611) | <.001 | <.001 | |
| Adjusted | .294 | ||||||
| Male | 1.000 | 0.631 (0.314, 1.268) | .180 | 0.704 (0.270, 1.835) | .446 | .518 | |
| Female | 1.000 | 1.409 (0.819, 2.425) | .198 | 0.704 (0.357, 1.388) | .288 | .308 | |
| β-cryptoxanthin | |||||||
| Unadjusted | .671 | ||||||
| Male | 1.000 | 1.111 (0.638,1.935) | .690 | 1.053 (0.717,1.547) | .775 | .778 | |
| Female | 1.000 | 0.869 (0.540,1.399) | .536 | 0.889 (0.565,1.397) | .583 | .557 | |
| Adjusted | .870 | ||||||
| Male | 1.000 | 1.057 (0.469, 2.383) | .885 | 1.128 (0.526, 2.420) | .741 | .743 | |
| Female | 1.000 | 1.025 (0.425, 2.470) | .954 | 1.546 (0.600, 3.984) | .343 | .364 | |
| Lutein/zeaxanthin | |||||||
| Unadjusted | .020 | ||||||
| Male | 1.000 | 1.096 (0.665,1.808) | .697 | 1.036 (0.707,1.519) | .844 | .848 | |
| Female | 1.000 | 0.961 (0.610,1.515) | .853 | 0.510 (0.310,0.837) | .012 | .013 | |
| Adjusted | .900 | ||||||
| Male | 1.000 | 0.583 (0.311, 1.095) | .088 | 1.011 (0.409, 2.499) | .980 | .861 | |
| Female | 1.000 | 0.543 (0.228, 1.294) | .155 | 0.913 (0.378, 2.209) | .830 | .773 | |
| Lycopene | |||||||
| Unadjusted | .014 | ||||||
| Male | 1.000 | 1.086 (0.619,1.905) | .756 | 1.128 (0.752,1.693) | .532 | .527 | |
| Female | 1.000 | 0.975 (0.676,1.405) | .884 | 0.563 (0.416,0.760) | .001 | .002 | |
| Adjusted | .299 | ||||||
| Male | 1.000 | 0.709 (0.307, 1.637) | .395 | 0.634 (0.302, 1.331) | .210 | .213 | |
| Female | 1.000 | 0.614 (0.237, 1.592) | .293 | 0.309 (0.117, 0.820) | .022 | .027 |
CI = confidence Interval, OR = odd ratio.
Adjusted Model: Adjusted for age, race/ethnicity, educational level, poverty-income ratio, body mass index, waist circumference, physical activity, sedentary behavior, smoking status, hypertension, diabetes mellitus, metabolic syndrome, ALT, AST, γ-GT, TG, LDL-C, FINS, FPG, HbA1c, Hs-CRP, and total calories.
3.4. Analysis of restricted cubic spline regression
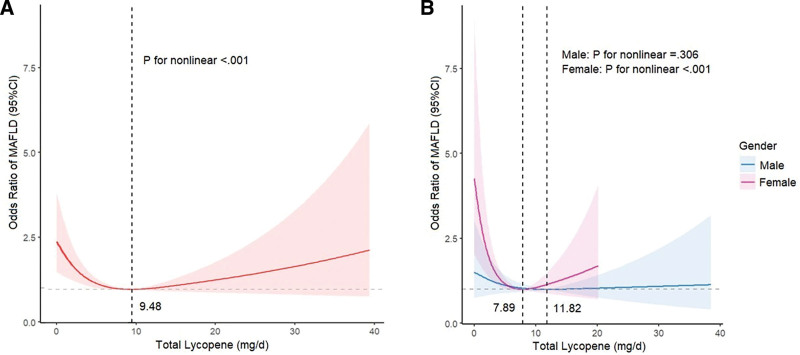
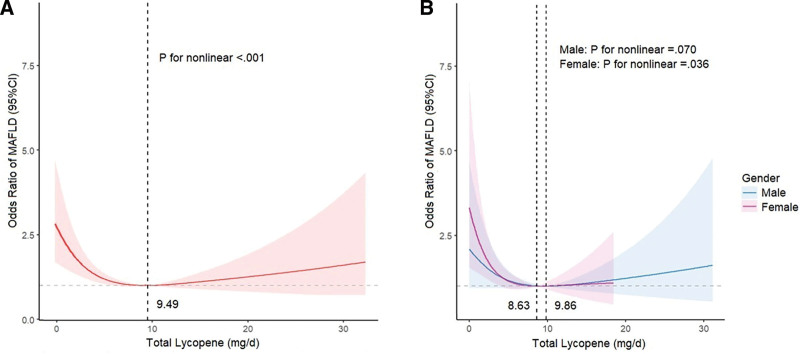
In restricted cubic spline regression analysis, we identified a noteworthy nonlinear association between the total intake of lycopene and MAFLD (P for nonlinear < .001, Fig. 3A) after adjusting for various covariates. Given the prominent U-shaped association between total lycopene intake and MAFLD risk, Figure 3A demonstrates a considerable risk reduction within the lower range of total lycopene intake, which reached the lowest risk around 9.48 mg/day and then increased thereafter. In the gender subgroup analysis, a notable nonlinear association between lycopene consumption and MAFLD persisted among females (P for nonlinear < .001, Fig. 3B) but not in males (P for nonlinear = .306, Fig. 3B). Despite slight differences in the results of sensitivity analysis, the nonlinear relationship remained consistent (Fig. 4).
Figure 3.
Restricted cubic spline depicts the association between total lycopene intake and MAFLD: (A) Restricted cubic spline analysis among all survey subjects, (B) restricted cubic spline analysis across gender subgroups. The red, blue, and purple lines represent ORs, and the red, blue, and purple transparent areas represent 95% CIs. Restricted cubic spline models are adjusted for age, gender, race/ethnicity, educational level, poverty-income ratio, body mass index, waist circumference, physical activity, sedentary behavior, smoking status, hypertension, diabetes mellitus, metabolic syndrome, ALT, AST, γ-GT, TG, LDL-C, FINS, FPG, HbA1c, Hs-CRP, and total calories. γ-GT = gamma-glutamyl transferase, ALT = alanine aminotransferase, AST = aspartate aminotransferase, CI = confidence interval, FINS = fasting insulin, FPG = fasting plasma glucose, HbA1c = glycohemoglobin, Hs-CRP = high-sensitivity C-reactive protein, LDL-C = low-density lipoprotein cholesterol, MAFLD = metabolic dysfunction-associated fatty liver disease, TG = triglyceride.
Figure 4.
Restricted cubic spline demonstrates the association between total lycopene intake and MAFLD in sensitivity analysis: (A) Restricted cubic spline analysis among all survey subjects, (B) restricted cubic spline analysis across gender subgroups. The red, blue, and purple lines represent ORs, and the red, blue, and purple transparent areas represent 95% CIs. Restricted cubic spline models are adjusted for age, gender, race/ethnicity, educational level, poverty-income ratio, body mass index, waist circumference, physical activity, sedentary behavior, smoking status, hypertension, diabetes mellitus, metabolic syndrome, ALT, AST, γ-GT, TG, LDL-C4, FINS, FPG, HbA1c, Hs-CRP, and total calories. γ-GT = gamma-glutamyl transferase, ALT = alanine aminotransferase, AST = aspartate aminotransferase, CI = confidence interval, FINS = fasting insulin, FPG = fasting plasma glucose, HbA1c = glycohemoglobin, Hs-CRP = high-sensitivity C-reactive protein, LDL-C = low-density lipoprotein cholesterol, MAFLD = metabolic dysfunction-associated fatty liver disease, TG = triglyceride.
4. Discussion
To elucidate the potential effect of carotenoid intake on the risk of MAFLD, a cross-sectional analysis was performed on 2722 participants from the NHANES 2017 to 2018. In this study, we demonstrated that intake of β-carotene, lutein/zeaxanthin, and lycopene may have protective effects against MAFLD. The restricted cubic spline analysis revealed that the aforementioned associations were primarily linear, whereas a nonlinear relationship between lycopene and MAFLD was detected, which manifested predominantly in females.
The multiple parallel hits hypothesis suggests that significant hepatic lipid accumulation and insulin resistance cause various metabolic alterations and lipotoxins production, which can lead to mitochondrial dysfunction, endoplasmic reticulum stress, and excessive reactive oxygen species generation, ultimately promoting the progression of MAFLD.[27,28] Several studies have shown that vitamin supplementation may protect liver tissue by reducing insulin resistance, lipid peroxidation, and fatty acid synthesis, and improving hepatic steatosis.[29,30] Carotenoids, the precursors of vitamin A, can regulate gene expression and immune responses and have potent antioxidant properties, suggesting a potential protective role in MAFLD.[31] In our study, no statistically significant correlation was found between the intake of α-carotene, β-cryptoxanthin, and MAFLD, despite earlier research indicating their potential in preventing NAFLD.[11,32] One possible explanation for this inconsistency is that past studies had a limited number of participants.
Our results showed that the association between β-carotene and MAFLD was relatively stable. Despite the lack of direct evidence, several research results support our findings. As a powerful anti-inflammatory and antioxidant micronutrient, β-carotene has shown promise in ameliorating hepatic steatosis and mitigating inflammation in individuals with NAFLD.[33] A mediation analysis study using data from the NHANES 2007 to 2010 revealed that dietary β-carotene may decrease the likelihood of NAFLD by suppressing inflammation.[34] Another case-control study, which recruited 24 control participants and 62 biopsy-proven NAFLD patients, revealed a notable decrease in serum β-carotene levels among NAFLD patients compared to the control group.[35] Furthermore, serum β-carotene levels gradually decreased with increasing histological severity of NAFLD, implying a potential protective effect of β-carotene on liver pathology.[35] Interestingly, Liu et al[12] discovered that a greater consumption of β-carotene was associated with a decrease in hepatic steatosis, although the relationship might be nonlinear. In contrast, our study suggested a potential linear relationship between β-carotene and MAFLD, which may be attributed to variations in the target study groups.
Lutein/zeaxanthin is mainly concentrated in the macula of the retina but is also distributed in the liver, adipose tissue, and brain, where it exerts potent antioxidant effects by neutralizing reactive oxygen species and directly quenching free radicals.[36] As shown in Figure 2, we have suggested the protective benefits of lutein/zeaxanthin intake on MAFLD. Similarly, previous research has indicated a negative correlation between the occurrence of NAFLD and plasma lutein/zeaxanthin in middle-aged and elderly Chinese populations, even after adjusting for potential confounders (all P values < .001).[37] Lutein supplementation has been demonstrated to alleviate hepatic lipid accumulation and improve insulin resistance in NAFLD rats induced by a high-fat diet.[38] In animal models fed a zeaxanthin-supplemented diet for 6 weeks, it was also observed that zeaxanthin treatment remarkably reduced hepatic lipid peroxidation and attenuated oxidative stress in a dose-dependent manner.[39] The underlying mechanism may be ascribed to the upregulation of proteins that are involved in lipid metabolism and insulin signal transduction, such as Sirtuin 1, peroxisome proliferators activated receptor-α, phosphatidylinositol 3-kinase, and glucose transporter-2, which is similar to the potential mechanism of vitamin K on MAFLD.[40]
As for lycopene, previous studies have yielded inconsistent findings concerning its effects. Numerous prior studies in animals and humans have suggested that it could prevent NAFLD by ameliorating hepatic steatosis and attenuating inflammation through its antioxidant properties.[6,13,41] Likewise, a recent meta-analysis concluded that higher lycopene levels are linked to reduced metabolic diseases such as NAFLD, type 2 diabetes mellitus, dyslipidemia, and metabolic syndrome.[42] However, a cohort study conducted in Japan found no significant relationship between NAFLD and serum lycopene levels.[43] What’s even more intriguing is that a Mendelian randomization study conducted by Chen et al[44] discovered that higher consumption of lycopene may lead to an elevated risk of NAFLD. However, it is remarkable that the restricted cubic spline analysis we performed showed evidence of a nonlinear relationship between total lycopene intake and MAFLD, which was particularly significant in females. As mentioned in a systemic review, the recommendations for dietary lycopene intake may be uncertain due to interindividual response variations in age, gender, disease state, and genetic makeup.[45] And differential effects of female hormones, such as estrogen and progesterone, on liver metabolism may explain the observed gender differences.[46,47] Future studies with appropriate consideration of hormonal status, gender, and sociocultural gender differences are warranted and may help us to comprehensively understand the risk, mechanisms, and therapeutic targets of MAFLD.
To the best of our knowledge, this study is the first to investigate the association between carotenoids and MAFLD. Our study has several advantages, including strict protocols and quality assurance measures, a nationally representative sample, and NHANES data with excellent quality. Additionally, we found potential protective effects of β-carotene, lutein/zeaxanthin, and lycopene against MAFLD, along with the potential existence of a nonlinear correlation between lycopene and MAFLD. However, our study has several limitations. First, the consumption of carotenoids was evaluated through two 24-hour dietary recall periods, which may not accurately mirror the true daily intake. Second, evidence of hepatic steatosis in MAFLD was determined using elastography rather than liver biopsy because liver biopsy is impractical in large-scale population studies. Moreover, despite our study being controlled for multiple covariates, the extent of their adjustment and residual confounding from unmeasured factors still require attention in observational studies. Finally, given that this cross-sectional study design may be subject to memory bias and is unable to establish cause-and-effect relationships, it is necessary to validate our findings through future studies that follow a prospective cohort design.
5. Conclusion
In summary, our findings suggested that an increased intake of β-carotene, lutein/zeaxanthin, and lycopene may reduce the risk of MAFLD. The association between MAFLD and total lycopene intake was nonlinear, especially in females. In the future, the causal relationship and precise mechanism between carotenoids and MAFLD should be further validated in large-scale prospective studies.
Acknowledgements
The authors thank all NHANES participants and staff for their valuable efforts and contributions. We also thank Zhang Jing (Second Department of Infectious Disease, Shanghai Fifth People’s Hospital, Fudan University) for his work on the NHANES database. His outstanding work, nhanesR package and webpage, makes it easier for us to explore the NHANES database.
Authors contributions
Conceptualization: Hang Zhang, Li Li.
Data curation: Hang Zhang, Li Li, Lei Jia.
Formal analysis: Hang Zhang, Lei Jia.
Investigation: Hang Zhang, Jinchun Liu.
Methodology: Hang Zhang, Li Li, Jinchun Liu.
Software: Hang Zhang, Li Li.
Validation: Hang Zhang, Jinchun Liu.
Visualization: Hang Zhang, Lei Jia.
Writing – original draft: Hang Zhang.
Writing – review & editing: Li Li, Jinchun Liu.
Supplementary Material
Abbreviations:
- γ-GT
- gamma-glutamyl transferase
- ALT
- alanine aminotransferase
- AST
- aspartate aminotransferase
- CAP
- controlled attenuation parameter
- CI
- confidence interval
- FINS
- fasting insulin
- FPG
- fasting plasma glucose
- HbA1c
- glycohemoglobin
- Hs-CRP
- high-sensitivity C-reactive protein
- LDL-C
- low-density lipoprotein cholesterol
- MAFLD
- metabolic dysfunction-associated fatty liver disease
- MEC
- mobile examination center
- NAFLD
- nonalcoholic fatty liver disease
- NHANES
- National Health and Nutrition Examination Survey
- PIR
- poverty-income ratio
- TG
- triglyceride
- VCTE
- vibration-controlled transient elastography
Supplemental Digital Content is available for this article.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
This study involving human participants was reviewed and approved by the National Center for Health Statistics Research Ethics Review Board (Protocol number: 2018-01). The patients/participants provided their written informed consent to participate in this study.
How to cite this article: Zhang H, Li L, Jia L, Liu J. Association between carotenoid intake and metabolic dysfunction-associated fatty liver disease among US adults: A cross-sectional study. Medicine 2023;102:51(e36658).
Contributor Information
Hang Zhang, Email: hang_shier@163.com.
Li Li, Email: lilililee@foxmail.com.
Lei Jia, Email: jialei@ihcams.ac.cn.
References
- [1].Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–9. [DOI] [PubMed] [Google Scholar]
- [2].Lim GEH, Tang A, Ng CH, et al. An observational data meta-analysis on the differences in prevalence and risk factors between MAFLD vs NAFLD. Clin Gastroenterol Hepatol. 2023;21:619–629.e7. [DOI] [PubMed] [Google Scholar]
- [3].Ciardullo S, Perseghin G. Prevalence of NAFLD, MAFLD and associated advanced fibrosis in the contemporary United States population. Liver Int. 2021;41:1290–3. [DOI] [PubMed] [Google Scholar]
- [4].Kim D, Konyn P, Sandhu KK, et al. Metabolic dysfunction-associated fatty liver disease is associated with increased all-cause mortality in the United States. J Hepatol. 2021;75:1284–91. [DOI] [PubMed] [Google Scholar]
- [5].Elvira-Torales LI, García-Alonso J, Periago-Castón MJ. Nutritional importance of carotenoids and their effect on liver health: a review. Antioxidants (Basel). 2019;8:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Clugston RD. Carotenoids and fatty liver disease: current knowledge and research gaps. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158597. [DOI] [PubMed] [Google Scholar]
- [7].Aune D, Keum N, Giovannucci E, et al. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: a systematic review and dose-response meta-analysis of prospective studies. Am J Clin Nutr. 2018;108:1069–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhai T, Li S, Hu W, et al. Potential micronutrients and phytochemicals against the pathogenesis of chronic obstructive pulmonary disease and lung cancer. Nutrients. 2018;10:813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hu Y, Cai X, Zhang N, et al. Relation between dietary carotenoid intake, serum concentration, and mortality risk of CKD patients among US adults: National Health and Nutrition Examination Survey 2001-2014. Front Med (Lausanne). 2022;9:871767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Beydoun MA, Beydoun HA, Fanelli-Kuczmarski MT, et al. Association of serum antioxidant vitamins and carotenoids with incident Alzheimer disease and all-cause dementia among US adults. Neurology. 2022;98:e2150–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Christensen K, Lawler T, Mares J. Dietary carotenoids and non-alcoholic fatty liver disease among US adults, NHANES 2003-2014. Nutrients. 2019;11:1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liu X, Shen H, Chen M, et al. Clinical relevance of vitamins and carotenoids with liver steatosis and fibrosis detected by transient elastography in adults. Front Nutr. 2021;8:760985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhu Y, Liu R, Shen Z, et al. Combination of luteolin and lycopene effectively protect against the “two-hit” in NAFLD through Sirt1/AMPK signal pathway. Life Sci. 2020;256:117990. [DOI] [PubMed] [Google Scholar]
- [14].Gofton C, Upendran Y, Zheng MH, et al. MAFLD: how is it different from NAFLD? Clin Mol Hepatol. 2023;29:S17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Patel CJ, Pho N, McDuffie M, et al. A database of human exposomes and phenomes from the US National Health and Nutrition Examination Survey. Sci Data. 2016;3:160096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Barr RG, Ferraioli G, Palmeri ML, et al. Elastography assessment of liver fibrosis: society of radiologists in ultrasound consensus conference statement. Radiology. 2015;276:845–61. [DOI] [PubMed] [Google Scholar]
- [17].Hashemi SA, Alavian SM, Gholami-Fesharaki M. Assessment of transient elastography (FibroScan) for diagnosis of fibrosis in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Caspian J Intern Med. 2016;7:242–52. [PMC free article] [PubMed] [Google Scholar]
- [18].Pu K, Wang Y, Bai S, et al. Diagnostic accuracy of controlled attenuation parameter (CAP) as a non-invasive test for steatosis in suspected non-alcoholic fatty liver disease: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Centers for Disease Control and Prevention. NHANES 2017-2018 procedure manuals. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/manuals.aspx?BeginYear=2017. [access date May 31, 2023].
- [20].Karlas T, Petroff D, Sasso M, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022–30. [DOI] [PubMed] [Google Scholar]
- [21].Stebbins RC, Noppert GA, Aiello AE, et al. Persistent socioeconomic and racial and ethnic disparities in pathogen burden in the United States, 1999–2014. Epidemiol Infect. 2019;147:e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kyu HH, Bachman VF, Alexander LT, et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ. 2016;354:i3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. J Hypertens. 2020;38:982–1004. [DOI] [PubMed] [Google Scholar]
- [24].American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S14–31. [DOI] [PubMed] [Google Scholar]
- [25].Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. [DOI] [PubMed] [Google Scholar]
- [26].Georgoulis M, Kontogianni MD, Margariti A, et al. Associations between dietary intake and the presence of the metabolic syndrome in patients with non-alcoholic fatty liver disease. J Hum Nutr Diet. 2015;28:409–15. [DOI] [PubMed] [Google Scholar]
- [27].Clare K, Dillon JF, Brennan PN. Reactive oxygen species and oxidative stress in the pathogenesis of MAFLD. J Clin Transl Hepatol. 2022;10:939–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–46. [DOI] [PubMed] [Google Scholar]
- [29].Xie ZQ, Li HX, Tan WL, et al. Association of serum vitamin C with NAFLD and MAFLD among adults in the United States. Front Nutr. 2022;8:795391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Barchetta I, Cimini FA, Cavallo MG. Vitamin D and metabolic dysfunction-associated fatty liver disease (MAFLD): an update. Nutrients. 2020;12:3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Milani A, Basirnejad M, Shahbazi S, et al. Carotenoids: biochemistry, pharmacology and treatment. Br J Pharmacol. 2017;174:1290–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ota T. Prevention of NAFLD/NASH by astaxanthin and β-cryptoxanthin. Adv Exp Med Biol. 2021;1261:231–8. [DOI] [PubMed] [Google Scholar]
- [33].Yilmaz B, Sahin K, Bilen H, et al. Carotenoids and non-alcoholic fatty liver disease. Hepatobiliary Surg Nutr. 2015;4:161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chen Y, Wu M, Chen F, et al. Potential role of inflammation in relation to dietary sodium and β-carotene with non-alcoholic fatty liver disease: a mediation analysis. Nutr Diabetes. 2022;12:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wang L, Ding C, Zeng F, et al. Low levels of serum β-carotene and β-carotene/retinol ratio are associated with histological severity in nonalcoholic fatty liver disease patients. Ann Nutr Metab. 2019;74:156–64. [DOI] [PubMed] [Google Scholar]
- [36].Bernstein PS, Li B, Vachali PP, et al. Lutein, zeaxanthin, and meso-zeaxanthin: the basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog Retin Eye Res. 2016;50:34–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cao Y, Wang C, Liu J, et al. Greater serum carotenoid levels associated with lower prevalence of nonalcoholic fatty liver disease in Chinese adults. Sci Rep. 2015;5:12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Qiu X, Gao DH, Xiang X, et al. Ameliorative effects of lutein on non-alcoholic fatty liver disease in rats. World J Gastroenterol. 2015;21:8061–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chamberlain SM, Hall JD, Patel J, et al. Protective effects of the carotenoid zeaxanthin in experimental nonalcoholic steatohepatitis. Dig Dis Sci. 2009;54:1460–4. [DOI] [PubMed] [Google Scholar]
- [40].Wang X, Zhang W, Huang J, et al. The relationship between vitamin K and metabolic dysfunction-associated fatty liver disease among the United States population: National Health and Nutrition Examination Survey 2017-2018. Front Nutr. 2023;10:1086477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Xiao ML, Chen GD, Zeng FF, et al. Higher serum carotenoids associated with improvement of non-alcoholic fatty liver disease in adults: a prospective study. Eur J Nutr. 2019;58:721–30. [DOI] [PubMed] [Google Scholar]
- [42].Iqbal WA, Mendes I, Finney K, et al. Reduced plasma carotenoids in individuals suffering from metabolic diseases with disturbances in lipid metabolism: a systematic review and meta-analysis of observational studies. Int J Food Sci Nutr. 2021;72:879–91. [DOI] [PubMed] [Google Scholar]
- [43].Sugiura M, Nakamura M, Ogawa K, et al. High serum carotenoids are associated with lower risk for developing elevated serum alanine aminotransferase among Japanese subjects: the Mikkabi cohort study. Br J Nutr. 2016;115:1462–9. [DOI] [PubMed] [Google Scholar]
- [44].Chen L, Fan Z, Sun X, et al. Diet-derived antioxidants and nonalcoholic fatty liver disease: a Mendelian randomization study. Hepatol Int. 2023;17:326–38. [DOI] [PubMed] [Google Scholar]
- [45].Böhm V, Lietz G, Olmedilla-Alonso B, et al. From carotenoid intake to carotenoid blood and tissue concentrations - implications for dietary intake recommendations. Nutr Rev. 2021;79:544–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lonardo A, Nascimbeni F, Ballestri S, et al. Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatology. 2019;70:1457–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yang JD, Abdelmalek MF, Guy CD, et al. Patient sex, reproductive status, and synthetic hormone use associate with histologic severity of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2017;15:127–131.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.