FIGURE 1.
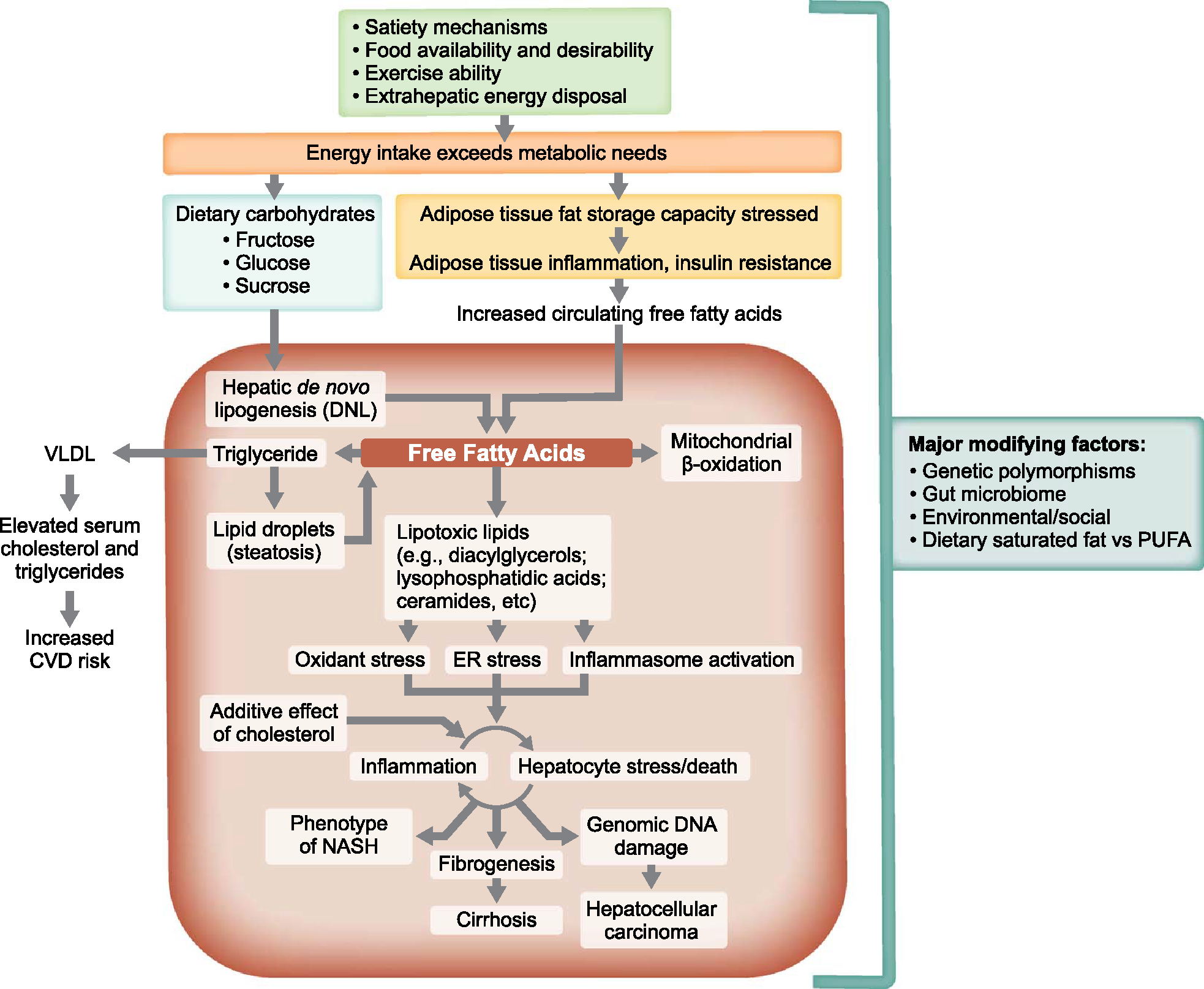
Pathogenic drivers of NAFLD as therapeutic targets. Overview of the major mechanisms that lead to the phenotype of NASH and its consequences, many of which can be leveraged therapeutically. Not shown are the many areas where genetic polymorphisms may play a role and where important modifying factors such as cholesterol, types of dietary fats consumed [saturated vs. polyunsaturated fatty acid (PUFA)], the gut microbiome, uric acid, and periodic hypoxia (sleep apnea) may also influence these pathways. A primary disease driver may be an oversupply of fat to adipocytes such that their ability to store triglyceride without inducing cell stress is exceeded, which activates inflammatory pathways and causes insulin resistance. Understanding the major drivers of NASH facilitates the rational development of therapies for patients with NASH. Specific sites of intervention that might prevent or resolve NASH include interventions that modulate food intake (eg, portion sizes, bariatric surgery, satiety regulators), increase energy disposal (eg, exercise, thermogenesis), improve adipocyte insulin sensitivity [eg, peroxisome proliferator-activated receptor (PPAR)γ ligands], impair de novo lipogenesis (eg, acetyl-coenzyme A carboxylase and fatty acid synthase inhibitors), increase hepatic oxidative metabolism (PPARα ligands and thyroid hormone receptor beta agonists), and attenuate inflammation, cell death, and fibrogenesis. Therapeutic agents affecting multiple metabolic pathways throughout the body with potential beneficial effects on the liver include peptide hormone analogs (eg, analogs of fibroblast growth factor-19, fibroblast growth factor-21, glucagon-like peptide-1, gastric inhibitory peptide, glucagon) and nuclear receptor ligands such as drugs that target PPARα, PPARδ, PPARγ, thyroid hormone receptor β, and farnesoid X receptor. Abbreviations: ER, endoplasmic reticulum; CVD, cardiovascular disease.
