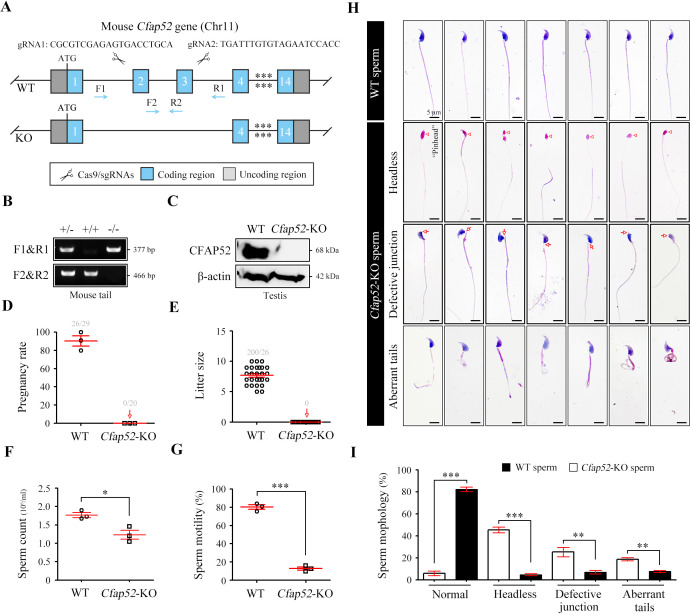
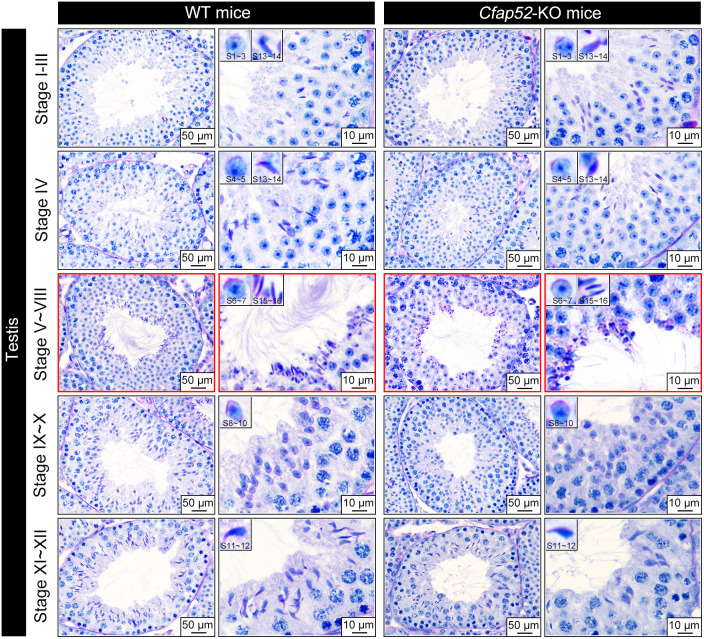
Figure 4. Cfap52 deficiency in mice leads to male sterility, with spermatozoa showing defective head-tail connections and flagella.
(A) Schematic illustration of the targeting strategy for generating Cfap52-KO mice by using CRISPR/Cas9 technology. A detailed procedure is described in the Materials and methods. (B) Representative results of PCR-based genotyping using mouse tail DNA. (C) Immunoblotting of CFAP52 was performed in the testis protein lysates of WT mice and Cfap52-KO mice. β-actin served as a loading control. Western blotting experiments were repeated three times with similar results. (D, E) Fertility assessment experiments were performed on three adult Cfap52-KO male mice and three WT male littermates for 2 months. Of the 20 female mice mated with Cfap52-KO male mice, no pregnancy was observed. (F, G) Sperm counts were counted with a fertility counting chamber under a light microscope, and total motility was assessed by a computer-assisted sperm analysis (CASA) system. Data are presented as the mean ± SEM (n=3 each group), Student’s t test, *p<0.05; ***p<0.001. (H) Morphological analyses of spermatozoa in WT mice and Cfap52-KO mice by Papanicolaou staining. First row, normal morphology of spermatozoa from WT mice; second row, headless spermatozoa in Cfap52-KO mice; third row, spermatozoa with defective head-tail connection in Cfap52-KO mice; fourth row, short-tailed spermatozoa in Cfap52-KO mice. The arrowheads indicate the ‘pinhead’, and the arrows indicate the defective head-tail connection. Scale bars, 5 μm. (I) Percentage of spermatozoa with normal morphology and each type of defect in WT mice and Cfap52-KO mice. At least 100 spermatozoa were counted for each mouse. Data are presented as the mean ± SEM (n=3 each group), Student’s t test, **p<0.01; ***p<0.001.