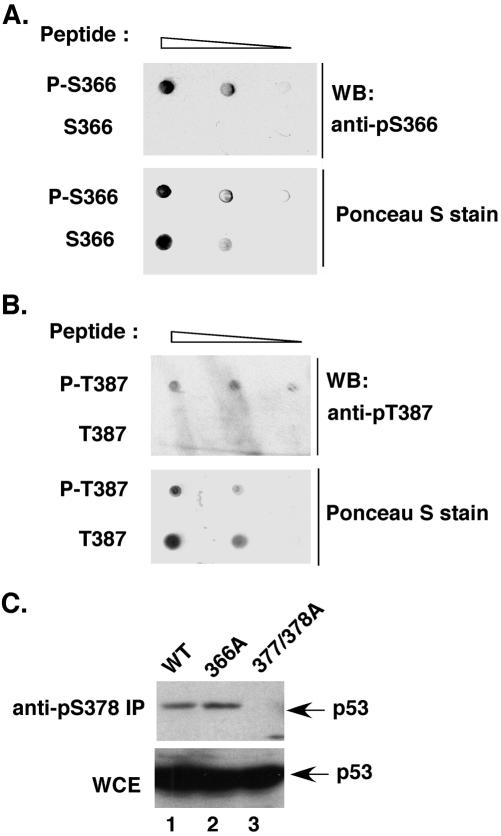
Figure 3.
Specificity of the anti-phospho-Ser and anti-phospho-Thr antibodies. The phosphopeptides and unphosphorylated peptides were spotted on nitrocellulose membranes in a series of two-fold dilutions starting from 250 ng. After Ponceau S staining to confirm equal loading, the membrane was incubated with either anti-phospho-Ser366 antibody (A) or anti-phospho-Thr387 antibody (B), as for Western analysis. Both antibodies specifically recognized the corresponding phosphopeptide but not the unphosphorylated peptide. (C) The anti-phospho-Ser378 antibody specifically immunoprecipitates WT p53 and the 366A mutant but not the 377/378A mutant. H1299 cells were transfected with the indicated p53 constructs. p53 in the lysates was immunoprecipitated with the antiphospho-Ser378 antibody and detected by Western analysis by using the p53-specific antibodies PAb1801 and DO1.