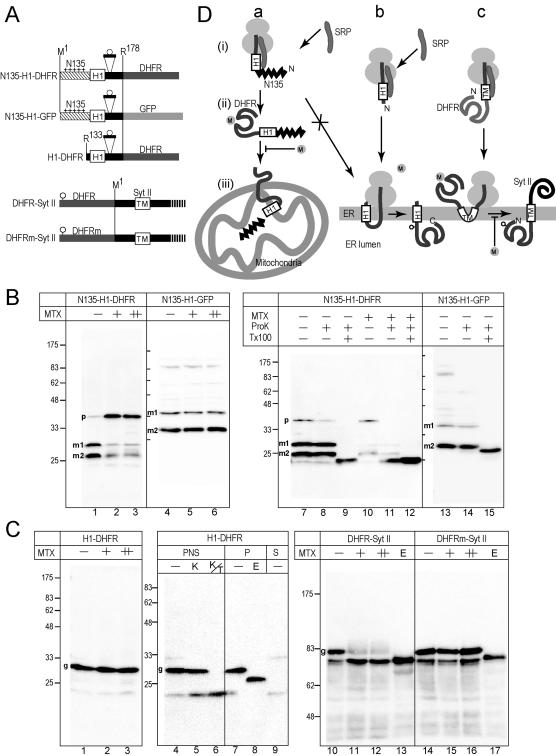
Figure 6.
Posttranslational translocation of the DHFR domain through the membrane is inhibited by MTX in COS7 cells. (A) The 178-residue N-terminal segment, which contains glycosylation sequence of nine residues, was fused to DHFR or GFP. In the third construct, the N-terminal 132 residues were deleted. In the last two constructs, the DHFR domains (DHFR and DHFRm) containing a glycosylation site (circle) were fused to SytII at Arg2. DHFRm denotes a DHFR mutant that does not bind MTX. (B) The constructs were expressed in COS7 cells in the presence of MTX (+, 10 μM; ++, 50 μM). As a mock control, the same volume of DMSO was added (–). Total cell lysate were subjected to immunoblotting analysis by using anti-DHFR or anti-GFP antibodies (lanes 1–6). Postnuclear supernatants were subjected to ProK treatment in the absence (–) or presence (+) of Tx100. Two processed mature forms (m1 and m2) and a precursor form (p) are indicated. (C) The constructs were expressed in COS7 cells in the presence or absence of MTX. Whole cell-lysate was analyzed by immunoblotting by using anti-SytII or anti-DHFR antibodies. Aliquots were treated with Endo H before the analysis (E lane). For ProK treatment, postnuclear supernatant was used (lanes 4–6). For Endo H treatment, the membrane precipitate fraction was used (lanes 7 and 8). Postmicrosomal supernatant fraction also was analyzed (S). In the case of SytII-related constructs, 12 and 36 μM MTX were used (+ and ++ lanes, respectively). (D) Schematic presentation of the effect of MTX. In the presence of N135, cotranslational integration of the H1 segments into the ER membrane was suppressed and posttranslational mitochondrial import was achieved (a). The cotranslational translocation of the DHFR domain across the ER membrane was not inhibited by MTX (b), whereas the translocation of the prefolded N-terminal DHFR domain was completely inhibited (c).