Abstract
Phagosomes undergo multiple rounds of fusion with compartments of the endocytic pathway during the course of maturation. Despite the insertion of vast amounts of additional membrane, the phagosomal surface area remains approximately constant, implying active ongoing fission. To investigate the mechanisms underlying phagosomal fission we monitored the fate of Fcγ receptors (FcγR), which are known to be cleared from the phagosome during maturation. FcγR, which show a continuous distribution throughout the membrane of nascent phagosomes were found at later times to cluster into punctate, vesicular structures, before disappearing. In situ photoactivation of receptors tagged with a light-sensitive fluorescent protein revealed that some of these vesicles detach, whereas others remain associated with the phagosome. By fusing FcγR to pH-sensitive fluorescent proteins, we observed that the cytoplasmic domain of the receptors enters an acidic compartment, indicative of inward budding and formation of multivesicular structures. The topology of the receptor was confirmed by flow cytometry of purified phagosomes. Phagosomal proteins are ubiquitylated, and ubiquitylation was found to be required for formation of acidic multivesicular structures. Remarkably, proteasomal function is also involved in the vesiculation process. Preventing the generation of multivesicular structures did not impair the acquisition of late endosomal and lysosomal markers, indicating that phagosomal fusion and fission are controlled separately.
INTRODUCTION
Phagocytosis is one of the earliest responses of the innate immune system to invasion by pathogens. Peptides derived from the degradation of microorganisms internalized by phagocytosis are routed to the cell surface together with the MHC complex for antigen presentation and stimulation of adaptive immunity. Phagocytosis is initiated by recognition of the pathogen by host cell receptors that trigger its engulfment into a specialized vacuole called the phagosome. Nascent phagosomes have a composition that resembles that of the plasmalemma and are unable to digest the vacuolar contents. This capability is acquired by remodelling of the phagosomal membrane and contents through a complex series of fusion events with compartments of the endocytic and secretory pathways (Desjardins et al., 1997). The components delivered to maturing phagosomes include acid hydrolases, as well as vacuolar-type ATPases that are responsible for acidification of the phagosomal lumen. This maturation process culminates in the formation of the phagolysosome, a highly acidic organelle (pH <5.5) where most of the bacterial killing and degradation occurs. Understanding the molecular mechanisms of phagosome maturation is critical, because a number of pathogens survive inside host cells through subversion of this process (Rosenberger and Finlay, 2003).
Although the fusion events that characterize maturation are important, remodeling of the phagosome also requires the concomitant loss of membrane and luminal components, which occurs through budding (fission) of vesicles (Pitt et al., 1992). This process mediates the salvage of valuable plasmalemmal components that are recycled to the cell surface, ensures that the maturing phagosome maintains a near constant size despite ongoing fusion events, and may contribute to the termination of signals to facilitate progression along the maturation pathway.
In addition, the phagosome is known to function as a complete antigen-processing compartment and fission of vesicles is required in order for MHC class II-antigen complexes derived from ingested pathogens to be presented at the cell surface (Ramachandra et al., 1999).
Despite the varied and important roles played by budding during phagosomal maturation, surprisingly little is known about its mechanisms. A number of coat complexes are thought to serve this function in endosomes, including COPI, COPII, and clathrin. However, earlier work revealed that omission of ε-COP, expected to impair the function of the COPI complex, only modestly decreased the clearance of transferrin receptors from the phagosomal membrane, with no discernible effect on the removal of Fc receptors or on the overall ability of phagosomes to mature (Botelho et al., 2000). Similarly, expression of dominant-negative forms of dynamin that impair clathrin-mediated endocytosis had little effect on transferrin receptor clearance from phagosomes or on their fusion with late endosome/lysosomes (Tse et al., 2003).
Recently, a group of protein complexes termed ESCRT (endosomal sorting complex required for transport) that mediate delivery of endosomal cargo to vesicles has been identified. The three known ESCRT complexes function in sequence to sort endosomal proteins into the luminal vesicles of multivesicular bodies (Katzmann et al., 2001). Sorting of proteins by ESCRT requires ubiquitylation of the target proteins. The recognition of ubiquitylated cargo is likely mediated by a subunit of ESCRT-I, the yeast protein Vps23 or its mammalian homologue Tsg101, both of which have a variant E2 domain that binds to ubiquitin. Disruption of this domain causes defects in the sorting and down-regulation of cell-surface receptors (Babst et al., 2000).
The multivesicular bodies (MVB) generated by the combined action of the ESCRT complexes are a subset of endosomes that have intraluminal vesicles and are thought to be important in receptor down-regulation and in antigen presentation (Katzmann et al., 2002). Because these functions are also central to the function of phagosomes, it is conceivable that ESCRT complexes may similarly assemble on their membrane and that a process analogous to MVB formation may contribute to maturation. In this regard, it is noteworthy that activation of the Fcγ receptors that mediate phagocytosis of IgG-opsonized particles was reported to trigger protein ubiquitylation (Booth et al., 2002). The role of ubiquitylation associated with phagocytosis remains unknown, but it could conceivably function to sort cargo during phagosome maturation. The purpose of this study, therefore, was to assess whether ubiquitin is involved in the remodeling of the phagosomal membrane. Specifically, we were interested in determining if structures analogous to MVB are generated from phagosomes and play an important role in receptor disposal and phagosomal maturation.
MATERIALS AND METHODS
Reagents and Antibodies
Fetal bovine serum (FBS), α-modified Earle's medium (MEM), DMEM, HEPES-buffered solution RPMI-1640 (HPMI), and phosphate-buffered saline (PBS) were from Wisent (St. Bruno, QC). Concanamycin A, G418, and MG-132 were from Calbiochem (La Jolla, CA). FM4-64 and LysoTracker Red were from Molecular Probes (Eugene, OR). Latex beads (3.1 μm diameter) were from Bangs Beads (Fishers, IN). Cy3-, Cy5-, phycoerythrin (PE)- and horseradish peroxidase (HRP)-conjugated secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA). Anti-LBPA antibodies were a kind gift from Dr. J. Gruenberg (Geneva, Switzerland). Anti-human CD32 (FcγRII) (clone AT10) was from Accurate Chemical and Scientific Corporation (Westbury, NY). FK2 and FK1 anti-ubiquitin antibodies were from Affiniti (Exeter, United Kingdom). Both antibodies are specific for ubiquitin and have been widely used in this field (Fujimuro et al., 1994; Haglund et al., 2003; Houde et al., 2003). Rabbit anti-GFP antibody was from Molecular Probes. Human IgG and all other reagents were from Sigma Aldrich (St. Louis, MO).
DNA Constructs
The plasmid encoding FcγRIIA-GFP was constructed by cloning a cDNA of FcγRIIA into pEGFP-N1 (Clontech, Palo Alto, CA) at HindIII and SacII sites. Photo-activatable GFP, which has a mutation in EGFP converting codon 203 from threonine to histidine (Patterson and Lippincott-Schwartz, 2002), was a gift from Dr. J. Lippincott-Schwartz (NIH, Bethesda, MD). PAGFP exhibits a 100-fold increase in fluorescence upon irradiation at 413 nm (Patterson and Lippincott-Schwartz, 2002), allowing for the selective induction of fluorescence at the desired site and time. FcγRIIA-PAGFP was constructed by replacing GFP with PAGFP at HindIII and SacII sites.
Cell Culture and Transfection
Ts20 and E36 cells were kindly provided by Dr. A. Schwartz (Washington University School of Medicine, St. Louis, MO). Chinese hamster ovary (CHO) and RAW264.7 cells were obtained from the American Type Culture Collection (Rockville, MD). Ts20 cells were grown in α-MEM with 5% FBS at 34°C in 5% CO2 under a humidified atmosphere. For inactivation of the E1 enzyme, ts20 cells were incubated in this medium at 42°C for 2 h. Transfections were performed with FuGene 6 (Roche Molecular Biochemicals, Indianapolis, IN) according to the manufacturer's instructions. Stable lines expressing FcγRIIA-GFP were selected with G418 at 0.5 mg/ml. Human monocytes were isolated by plating the lymphocyte/monocyte-rich layer of blood from healthy volunteers on coverslips bathed in DMEM with 5% FBS, incubating them at 37°C, followed by washing with sterile PBS and changing the medium daily for 5 d to remove platelets and lymphocytes.
Phagocytosis Assays
Latex beads were opsonized with 3.75 mg/ml human IgG for 1 h at 37°C. Cells grown on glass coverslips were changed to prewarmed serum-free medium and were overlaid with 25 μl of opsonized latex beads and incubated at 37°C to initiate phagocytosis. After 5–20 min of phagocytosis, particles that were not internalized were labeled by incubating with Cy5- or Cy3-conjugated anti-human IgG for 3 min on ice, after which time the medium was changed and the cells were returned to 37°C for the indicated time. For experiments using ts20 cells preincubated at 42°C, labeling of external particles was done at 37°C to minimize the temperature drop. Quantitation of FcγRIIA-GFP intensity and distribution, as well as LBPA, LysoTracker Red, and FM4-64 acquisition was limited to those phagosomes that had formed during the first 15–20 min (i.e., those not labeled by externally added anti-IgG).
In most experiments, phagocytosis was synchronized by centrifugation of the cells after the addition of beads at 1500 rpm for 1 min. When indicated, cells were pretreated with 10 μM MG-132 for 1.5–3 h. Lysosomes of RAW cells were prelabeled by incubation with FM4-64 (10 μM) for 1 h, followed by a 2.5-h chase in the absence of the dye. In cells that were also treated with MG-132, the proteasome inhibitor was added after a chase period of 1 h had elapsed. For labeling with LysoTracker Red, RAW cells were otherwise untreated (control) or were pretreated with MG-132 and allowed to internalize opsonized beads as described earlier. After a chase period of at least 50 min, cells were incubated with 50 nM LysoTracker Red for 5 min. For both experiments, live cells were analyzed by fluorescence microscopy to determine the percentage of FM4-64– or LysoTracker Red-positive phagosomes.
Phagosome Isolation
Phagosomes were isolated from RAW macrophages by sucrose density centrifugation as previously described (Desjardins et al., 1994). The purity of our preparation was tested by scanning and transmission electron microscopy and by enrichment in LAMP-1 and found to be comparable to that reported by Desjardins et al. (1994). Phagocytosis was synchronized by adding opsonized latex beads to cells in the cold for 20 min. After 12 min of phagocytosis, cells were washed several times with cold PBS before the addition of homogenization buffer. After isolation, equal amounts of phagosomes were used for analysis by measuring light scattering at 600 nm (Gotthardt et al., 2002).
Microscopy and Immunofluorescence
Immunofluorescence of FcγRII on human monocytes was performed by fixing cells in 3% paraformaldehyde followed by permeabilization in 0.1% Triton, blocking in 5% donkey serum, and then probing with mouse anti-human CD32 (clone AT10) at 1:100 for 1 h. After washing with PBS, samples were incubated with Cy-3-anti-mouse antibody (1:500) for 30 min and then washed and mounted on slides. Immunofluorescence of LBPA followed a similar protocol except that cells were permeabilized with 0.05% saponin and were blocked in PBS with 0.1% bovine serum albumin.
Immunofluorescence detection of ubiquitin was performed by first permeabilizing cells in ice-cold 0.05% saponin for 5 min followed by fixation in 3% paraformaldehyde, blocking in 5% donkey serum, and incubating with the FK2 anti-ubiquitin antibody at 1:100 in PBS with 0.05% saponin. After washing, samples were incubated with Cy-3-anti-mouse antibody (1:500 in PBS/saponin) for 30 min and then washed and mounted.
To assess the change in fluorescence of FcγRIIA-GFP upon addition of NH4Cl, cells were incubated in serum-free HPMI and 25 μl of opsonized latex beads added. After at least 40 min of phagocytosis, live cells in HPMI were analyzed by fluorescence microscopy using a Leica DM-IRB microscope (Deerfield, IL) adapted with a Micromax camera under the control of Improvision software (Lexington, MA). Images were acquired using identical settings before and after addition or removal of 10 mM NH4Cl. In some experiments 250 nM concanamycin A was added 25 min after the onset of phagocytosis and cells were returned to the incubator for another 15 min before analysis.
Photo-activation of FcγRIIA-PAGFP was accomplished using the Chameleon (Coherent, Santa Clara, CA) two-photon laser tuned to 800 nm. Analysis of the distribution and density of FcγRII, FcγRIIA-GFP, and FcγRIIA-PAGFP and the detection of LBPA on the phagosome was performed using an LSM510 laser scanning confocal microscope (Zeiss, Thornwood, NY) with a 100× oil immersion objective. Phagolysosome fusion was assessed using a Leica DM-IRB microscope with standard filter sets.
Flow Cytometry
Ts20 cells stably transfected with FcγRIIA-GFP were incubated at 34 or 42°C for 2 h before ingesting opsonized latex beads. After 1.5 h of phagocytosis, phagosomes were isolated by sucrose density centrifugation as above. Purified phagosomes were fixed in 4% paraformaldehyde for 30 min, blocked in 5% milk for 1 h, and then incubated in 100 mM glycine for 10 min. Phagosomes were either permeabilized with 0.1% Triton X-100 for 20 min at room temperature or left intact and then probed with rabbit anti-GFP for 1 h. After washes, the secondary antibody, phycoerythrin (PE)-conjugated goat anti-rabbit, was applied for 30 min. Phagosomes were passed several times through a 25-gauge needle to disrupt aggregates before flow cytometry. A minimum of 5000 phagosomes were analyzed for each condition in a FACS-Calibur cytometer (Becton-Dickinson, Mountain View, CA). Results were analyzed using FlowJo software (Tree Star Flow Jo, Ashland, OR).
Electron Microscopy
RAW cells were allowed to internalize IgG-opsonized RBC or latex beads for 5, 20, 45, or 60 min at 37°C. Cells were fixed in 2% glutaraldehyde in 0.1 M Sorenson's phosphate buffer, pH 7.2, for 5 min before being scraped off the coverslip and centrifuged. Fixation was continued at room temperature for at least a further 1 h. Cells were then postfixed in 1% OsO4 in phosphate buffer at room temperature for 2 h, stained en bloc for 1 h with 1% uranyl acetate in H2O, and then dehydrated and embedded in Epon resin. Sections (70–80 nm thick) were collected on copper grids and stained with uranyl acetate and lead citrate. Serial sections (80 nm apart) were also collected where specified. Sections were viewed using a FEI Tecnai (Hillsboro, OR) 20 electron microscope and images were captured using a Gatan Dualview camera (Pleasanton, CA).
Immunoblotting
Equal numbers of phagosomes were analyzed by SDS-PAGE using 12–15% polyacrylamide gels. Proteins were transferred to nitrocellulose membranes, blocked for 1 h in 5% milk in PBS with 0.05% Tween-20 (PBS/Tween), and then probed overnight with the primary antibody (at 1:500–1:1000) at 4°C. After washing in PBS/Tween, blots were incubated with HRP-conjugated secondary antibodies (1:2000–1:5000) for 30 min, washed vigorously, and then visualized by enhanced chemiluminescence (Amersham, Piscataway, NJ).
Statistics
Unless otherwise stated, all experiments were performed at least in triplicate. Chi square tests were used to compare the distribution of Fcγ receptors on phagosomes under various conditions. For comparisons of means, t tests were used. For both tests p < 0.05 was deemed significant.
RESULTS
Distribution of FcγRIIA during Phagosome Maturation
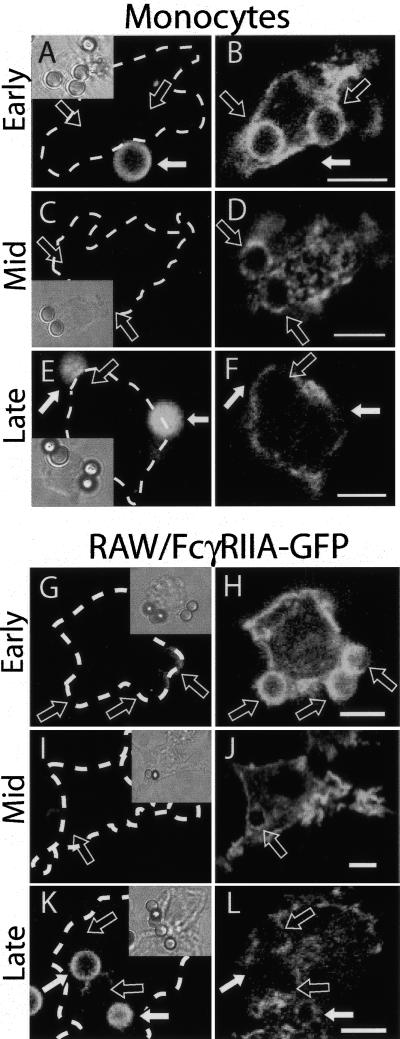
We analyzed the fate of Fcγ receptors as an index of phagosomal remodelling during phagocytosis. We chose to study FcγRIIA not only because it is abundant and widely expressed, but also because it consists of a single polypeptidic chain, unlike other FcγR species that require a separate γ subunit to mediate phagocytosis (Gessner et al., 1998). The distribution of endogenous FcγRIIA in human monocytes was defined at various times after phagocytosis of latex beads, using immunofluorescence. Shortly after particle ingestion (5–10 min after exposure to the opsonized particles), FcγRIIA appeared as an intense and continuous rim surrounding the bead (Figure 1, A and B). As phagosome maturation progressed, however, the receptor distribution became patchy (Figure 1, C and D), fainter, and ultimately undetectable (Figure 1, E and F). A similar pattern was observed when FcγRIIA fused to green fluorescent protein (GFP) was expressed in RAW264.7 macrophages (Figure 1, G–L) and even in transfected CHO cells (Figure 2, A–C), which are not professional phagocytes. Expression of FcγR was shown earlier to confer phagocytic capabilities to nonmyeloid cells, including CHO fibroblasts (Indik et al., 1995). Remarkably, such engineered phagocytes can recapitulate not only particle engulfment, but also most aspects of phagosomal maturation (Downey et al., 1999). We utilized these nonprofessional phagocytes because they have unique, advantageous features, including the presence of a single, defined type of phagocytic receptor and our ability to generate stable transfectants from these cells, but not from macrophages. We quantified the rate of loss of fluorescence from the phagosomal membrane in live CHO cells stably transfected with FcγRIIA-GFP by integration of digital images. The half-life of the fluorescent receptors at the phagosomal membrane was calculated to average 15 min (Figure 2D). Similar quantitation of RAW cells transiently transfected with FcγRIIA-GFP yielded a slightly longer half-life (unpublished data); it must be borne in mind that because RAW cells possess other types of Fcγ receptors that mediate phagocytosis of IgG-opsonized particles, the degree of engagement of the heterologously expressed FcγRIIA on any given phagosome is difficult to ascertain.
Figure 1.
Changes in FcγIIA receptor density and distribution during phagosome maturation. (A–F) Human monocytes were allowed to engulf IgG-opsonized latex beads for 5 min and beads that had not been internalized were labeled with Cy5-conjugated antihuman IgG (A, C, and E). The cells were then either fixed immediately (A and B) or chased for 5 (C and D) or 10 min (E and F) before fixation. After fixation, cells were immunostained for FcγRII using antihuman CD32. (G–L) RAW macrophages transfected with FcγRIIA-GFP were allowed to engulf IgG-opsonized latex beads for 10 min and beads that had not been internalized were labeled with Cy5-conjugated anti-human IgG (G, I, and K). The cells were then either fixed immediately (G and H) or chased for 10 (I and J) or 50 min (K and L) before fixation. Insets show corresponding differential interference contrast (DIC) images. In all panels, open arrows denote sealed phagosomes and solid white arrows indicate external beads. Images are representative of at least three separate experiments of each type. Size bars, 5 μm.
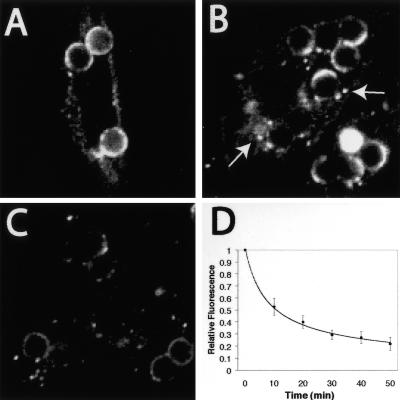
Figure 2.
Detection of FcγRIIA in vesicles following phagocytosis. (A–C) CHO cells stably transfected with FcγRIIA-GFP were allowed to engulf opsonized latex particles for 20 min and either fixed immediately (A) or chased for 20 (B) or 40 min (C) before fixation and analysis by confocal microscopy. Arrows in B denote fluorescent vesicles near phagosomes. (D) The loss of fluorescence from the phagosome over time was quantified in live CHO cells stably transfected with FcγRIIA-GFP. Confocal fluorescence images were acquired at regular intervals and the emission intensity was measured and is plotted as a fraction of the initial fluorescence. The contribution of photobleaching was negligible, because acquisition of images at similar intervals produced no significant loss of the fluorescence intensity of the plasmalemma in control cells that had not ingested beads. Data are means ± SE of at least 30 individual determinations from three separate experiments.
The loss of fluorescence observed in Figures 1 and 2 suggested that the receptors were being degraded in situ or were removed from the limiting membrane of the phagosome by fission/budding. Both explanations account for the gradual disappearance of FcγRIIA from the phagosome, but the latter is more readily compatible with the discontinuous pattern noted in the intermediate stages of maturation. Indeed, diminution of fluorescence from the phagosomal membrane was generally accompanied by the appearance of small fluorescent vesicles near the maturing phagosome (e.g., Figures 1D and 2B).
Fission of FcγRIIA-containing Vesicles from the Phagosome
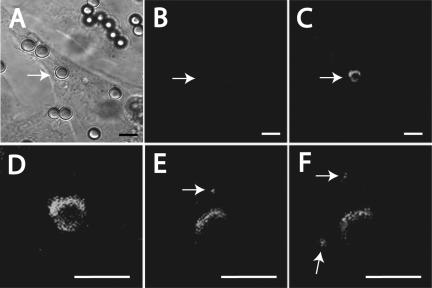
The vesicles containing FcγRIIA detected in the vicinity of phagosomes may have arisen from budding and fission of the limiting membrane of the phagosome. However, endocytosis of plasmalemmal FcγRIIA not engaged in phagocytosis could have equally generated fluorescent endomembrane vesicles. To rule out the latter possibility, we transfected CHO cells with a chimeric construct consisting of FcγRIIA fused to a photo-activatable mutant of GFP (PAGFP). PAGFP exhibits a 100-fold increase in fluorescence upon irradiation at 413 nm (Patterson and Lippincott-Schwartz, 2002), allowing for the selective induction of fluorescence at the desired site and time. We used irradiation of FcγRIIA-PAGFP to generate fluorescence and selectively visualize receptors present in early phagosomes, whereas plasmalemmal receptors remained virtually nonfluorescent and therefore undetectable. Figure 3, A–C, demonstrates that the FcγRIIA-PAGFP chimera is competent for induction of phagocytosis, because the presence of phagosomes could be readily established by differential interference contrast (DIC) microscopy, and that the fluorescence level of transfected but nonirradiated cells is negligible. After irradiation at 413 nm of the phagosome identified by the arrow, a bright and nearly continuous rim of FcγRIIA appeared around the opsonized bead, whereas the rest of the cell remained nonfluorescent. As a control, photoactivation of areas of the cell away from the phagosome (located previously by differential interference contrast microscopy) did not result in the generation of fluorescent vesicles (unpublished data). As in the case of the endogenous receptors, the activated phagosomal FcγRIIA-PAGFP became discontinuous and dim as the phagosome matured (Figure 3, D–F). Importantly, small fluorescent structures were observed to separate from the maturing phagosome, consistent with the occurrence of fission of vesicles from the limiting membrane of the phagosomal vacuole.
Figure 3.
Use of photoactivation to monitor FcγRIIA traffic in phagosomes. (A–F) CHO cells were transfected with FcγRIIA-PAGFP. Transfected cells expressing receptors were first identified by DIC based on their association with beads (A). The low fluorescence emitted by these cells before photoactivation was verified (B). The FcγRIIA-PAGFP associated with the phagosome indicated by the arrow was next activated using the Chameleon laser as described in Materials and Methods, and the appearance of fluorescence verified by confocal microscopy (C). (D–F) Magnified views of the same phagosome acquired shortly after activation (D) and 30 min (E) and 40 min (F) thereafter. Size bars, 5 μm.
Phagosome-associated FcγRIIA Is Sorted to a Multivesicular Compartment
Segregation of receptors from the phagosome could occur, most simply, by outward budding of vesicles that preserve the same topology as the phagosomal membrane, such that the intracellular tail of the receptors always faces the cytosol. However, as discussed in the introduction, inward budding of endomembranes has also been observed, yielding multivesicular bodies (MVB) with internal vesicles of reverse topology, i.e., with the receptor tails contained within the lumen of the MVB. We took advantage of the pH sensitivity of GFP to distinguish between these mechanisms. The rationale of these experiments is diagrammatically presented in Figure 4A. Briefly, in FcγRIIA-GFP the C-terminal fluorescent protein resides in the cytosol when the receptor is at the plasma membrane or in the membrane limiting the phagosome. The chimera therefore senses the cytosolic pH, which is near neutral, and should fluoresce brightly and be only modestly affected by small concentrations of ammonia. Vesicles generated by outward budding should maintain this behavior. In contrast, inward budding would deliver the tail of the receptors to the lumen of MVB. The luminal pH of components of the endocytic pathway, including MVB and the phagosome, is acidic and therefore expected to quench GFP fluorescence. Such quenching should be relieved by permeant weak bases such as ammonia (Figure 4A). It is noteworthy that internal vesicles of MVB have a finite permeability to H+, are not thought to have functional V-ATPases and would be devoid of ATP, and are therefore expected to have an acidic lumen.
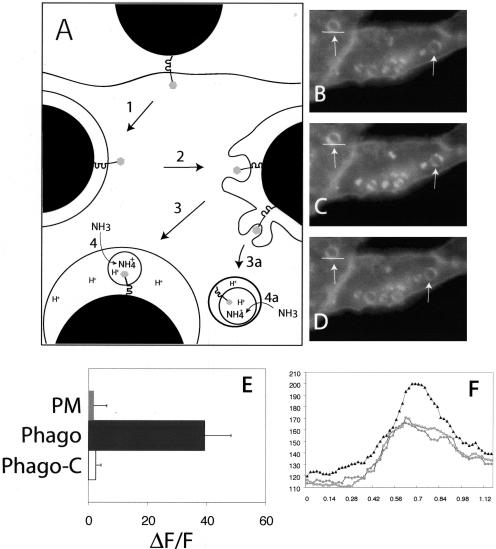
Figure 4.
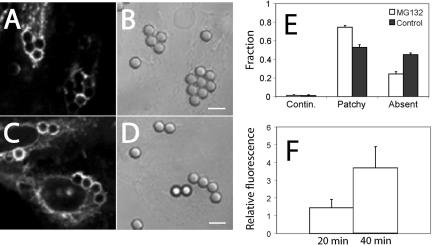
Translocation of the cytosolic tail of FcγRIIA to an acidic compartment during phagosome maturation. (A) Diagrammatic representation of the experimental protocol. Cells stably transfected with FcγRIIA-GFP were allowed to ingest opsonized latex beads (arrow 1). During the course of maturation the phagosomal membrane bearing FcγRIIA-GFP undergoes budding (2) and fission (3 and 3a). Fission results in the release of intraphagosomal vesicles (3) or the formation of multivesicular bodies containing FcγRIIA-GFP (3a). The lumen of the phagosome and of the MVB is acidic, leading to quenching of the fluorescence of GFP, which can be relieved by alkalinization induced by addition of ammonia (4 and 4a), added as NH4Cl. (B–D) Cells stably transfected with FcγRIIA-GFP were allowed to ingest opsonized latex beads for 45 min at 37°C. Fluorescence emission was acquired before (B) and after the addition of 10 mM NH4Cl (C). Another image (D) was acquired shortly after removal of NH4Cl. Representative phagosomes are denoted with arrows. Images are representative of three separate experiments. (E) Quantitation of fluorescence change induced by addition of NH4Cl in the immediate vicinity of the phagosome (Phago) and at the plasma membrane (PM). In separate experiments, cells prepared as above were treated with 250 nM concanamycin A for 15 min before image acquisition and addition of NH4Cl (Phago-C). Results are presented as the fluorescence change (ΔF) induced by NH4Cl normalized to the total initial fluorescence of the phagosome (F). Data are means ± SE of at least 50 individual determinations. (F) Scans of the fluorescence intensity of one of the phagosomes in B–D. The region denoted by the white line was scanned and is plotted in F to demonstrate the increase in fluorescence induced by ammonia. The ordinate shows the relative fluorescence intensity (RFI). Circles and squares represent fluorescence intensity before addition and after removal of NH4Cl, respectively, and black triangles represent the intensity in the presence of NH4Cl.
To determine the directionality of receptor budding, CHO cells stably transfected with FcγRIIA-GFP were allowed to engulf opsonized beads and, after allowing maturation for ≈40 min, were monitored by fluorescence microscopy. As expected, FcγRIIA-GFP fluorescence became patchy and dim over the course of phagosome maturation (Figure 4B). Addition of ammonia resulted in a marked and statistically significant (p < 0.001) increase in the intensity of the punctate fluorescence associated with and in the vicinity of the phagosomal membrane, but a much smaller change at the plasma membrane (Figure 4, C, E, and F). Subsequent removal of ammonia restored the original, lower levels of fluorescence, ruling out a nonspecific deleterious effect (Figure 4, D and F). To confirm that the ammonia-sensitive quenching of FcγRIIA-GFP fluorescence is due to its exposure to the acidic lumen of endocytic organelles, the cells were pretreated with concanamycin A (folimycin), a potent and specific inhibitor of vacuolar-type ATPases (Drose and Altendorf, 1997). Concanamycin A completely abrogated the increase in phagosome-associated fluorescence induced by ammonia (Figure 4E). These data are consistent with the notion that (at least a fraction of) FcγRIIA is delivered to the lumen of MVB during phagosomal maturation.
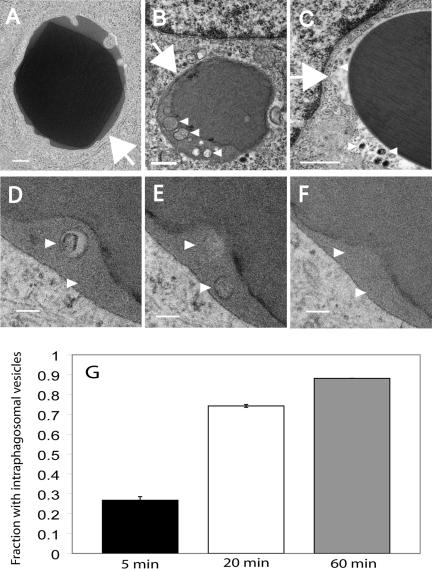
The presence of MVB in association with the maturing phagosome was confirmed by transmission electron microscopy (TEM). RAW macrophages were allowed to internalize opsonized sheep red blood cells or latex beads, followed by a 45-min maturation period before fixation. TEM revealed the presence of MVB in the immediate vicinity of the phagosomes, which may have been generated by fission from phagosomes. More importantly, after phagocytosis of either red blood cells or latex beads, numerous discrete vesicles were often observed within the phagosome lumen (Figure 5, B and C). In sections prepared for TEM circular profiles such as those in Figure 5, B and C, are generally interpreted as vesicles, but may result from transverse sections of invaginated tubules. To rule out the latter possibility, we analyzed serial sections. As documented in Figure 5, D–F, the structures found inside phagosomes are not continuous tubules but bona fide vesicles. Such vesicles are likely to have been generated by inward budding of the limiting membrane of the phagosomal vacuole. Thus, late phagosomes are multivesicular organelles. The proportion of phagosomes exhibiting internal vesicles increased sharply over time (Figure 5G), suggesting that phagosomes acquire internal vesicles as they mature. The differences in the abundance of vesicles were statistically significant (p < 0.02, for 5 min vs. 60 min and p < 0.05 for 20 min vs. 60 min).
Figure 5.
Detection of intraphagosomal vesicles by transmission electron microscopy (TEM). (A and B) RAW macrophages were allowed to ingest opsonized sheep red blood cells (A, B, and D–F) or latex beads (C), fixed at the indicated times, and processed for TEM. Phagocytosis was arrested by fixation after (A) 5 min; (B and D–F) 60 min; and (C) 45 min. Samples shown in D–F are serial sections of the same phagosome taken 80 nm apart. Arrows indicate the limiting membrane of the phagosome; arrowheads denote intraphagosomal vesicles. Bar, (A–C) 0.5 μm; (D–F) 0.2 μm. The proportion of phagosomes exhibiting internal vesicles was quantitated at the three different time points from two separate experiments (G). Error bars (which are too small to be visualized at 60 min), ±SE.
Ubiquitin Is Recruited to the Phagosome during Phagosome Maturation
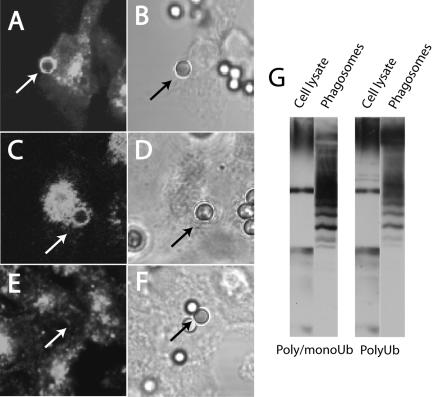
In endosomes, sorting of cargo into multivesicular bodies has been shown to require ubiquitylation (Katzmann et al., 2002). We therefore tested whether phagosome-associated proteins are ubiquitylated. Phagosomes isolated from RAW cells were separated by SDS-PAGE and the presence of ubiquitylated proteins was tested by immunoblotting. As shown in Figure 6G, a variety of bands were identified in the phagosomes, which were enriched in ubiquitylated proteins compared with the whole-cell lysate. Different patterns were observed when blotting with an antibody that recognizes poly-ubiquitylated proteins compared with another one that detects both single and multiple ubiquitin repeats, implying that both mono- and poly-ubiquitylated proteins exist on the phagosomal membrane. Abundant ubiquitylation was also detected on phagosomes by immunostaining, as shown in Figure 6A for cells transfected with FcγRIIA-GFP.
Figure 6.
Ubiquitin is recruited to the phagosome during maturation. (A and B) CHO cells stably transfected with FcγRIIA-GFP were allowed to internalize opsonized latex particles for 20 min. The cells were fixed and permeabilized and immunostained with an antibody that recognizes both poly- and mono-ubiquitin (A). Corresponding DIC image (B). (C–F) Ts20 cells stably transfected with FcγRIIA-GFP were either maintained at 34°C (C and D) or were preincubated at 42°C for 2 h (E and F) before phagocytosis and immunostaining as above. (C–E) ubiquitin immunostaining; (D–F) corresponding DIC images. Arrows point to phagosomes. (G) RAW macrophages were allowed to ingest opsonized latex beads for 12 min at 37°C before homogenization and isolation of phagosomes by sucrose density centrifugation. Purified phagosomes were immunoblotted with an antibody that reacts with both poly- and mono-ubiquitin and another one for poly-ubiquitin alone. An equivalent amount of protein from the starting whole-cell lysate was used as reference. Blots and images are representative of at least three similar experiments of each type.
Impairment of Ubiquitylation Alters the Fate of FcγRIIA in Phagosomes
We proceeded to test the role of ubiquitin in phagosomal remodelling. To this end, we took advantage of the ts20 line of hamster fibroblasts. These cells bear a temperature-sensitive mutation that results in the rapid inactivation of the E1 ubiquitin-activating enzyme when incubated at ≥40°C (Handley-Gearhart et al., 1994). Like CHO cells, ts20 fibroblasts grown at a permissive temperature (34°C) and transfected with FcγR acquire the ability to engulf IgG-opsonized particles (e.g., Figure 6, C and D). As in the other cell types described above, the phagosomes formed by ts20 cells are richly endowed with ubiquitylated proteins. Incubation at a restrictive temperature did not impair the ability of the cells to internalize particles, but greatly diminished the density of ubiquitylated proteins associated with the phagosome (Figure 6, E and F). This provided a suitable model to test whether ubiquitylation is an essential requirement for phagosomal maturation and for delivery of FcγRIIA to MVB in particular.
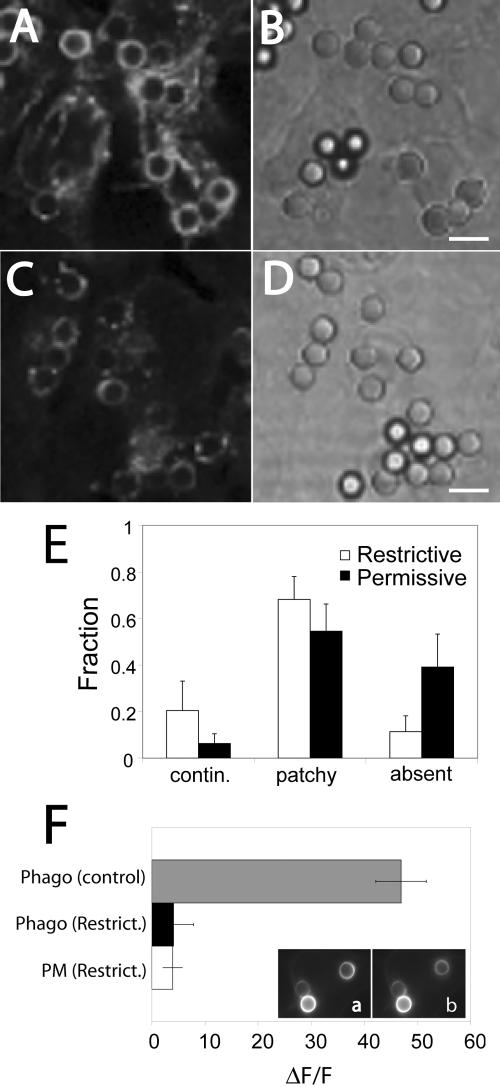
In ts20 cells cultured at 34–37°C (i.e., ubiquitination-competent), phagosomal FcγRIIA-GFP became patchy and decreased in fluorescence over time (Figure 7, C–E), as described for RAW and CHO cells. Impairment of ubiquitylation by preincubating the cells for 2 h at restrictive temperatures extended the residence time of FcγRIIA-GFP on the phagosomal membrane. The fluorescence from the receptors remained significantly more continuous and was brighter than observed at the permissive temperature (Figure 7, A and E). After 40 min, the residual fluorescence in E1-deficient cells was 4.1 ± 1.6-fold brighter than in ubiquitylation-competent cells (p < 0.05; unpublished data). This decrease in the rate of receptor processing is the result of inactivation of the E1 ubiquitin-activating enzyme and not a direct, nonspecific consequence of the temperature change. This was verified by transfecting E36 cells with FcγRIIA-GFP. E36 is the parental line that was mutagenized to generate the ts20 clone. Transfected E36 cells were preincubated for 2 h at 34 or 42°C and the receptor distribution was analyzed as described above. The rate and extent of receptor patchiness and dimming under both conditions were indistinguishable (unpublished data).
Figure 7.
Impairing ubiquitination prevents the loss of FcγRIIA from the phagosome. Ts20 cells stably transfected with FcγRIIA-GFP were either maintained at the permissive temperature (34°C; C and D) or were preincubated at a restrictive temperature (42°C) for 2 h (A and B) before phagocytosis of opsonized beads for 40 min at 37°C. The cells were then fixed and the distribution of FcγRIIA-GFP analyzed by laser confocal microscopy (A and C). Corresponding DIC images appear in B and D. (E) The fluorescence pattern of each phagosome was categorized as being continuous around the phagosome, patchy (discontinuous) or absent from the phagosome. The fraction of each type is shown in E for both conditions. (F) Quantitation of fluorescence change induced by addition of NH4Cl in the immediate vicinity of the phagosome (Phago) and at the plasma membrane (PM). Ts20 cells stably transfected with FcγRIIA-GFP were incubated at the permissive (control) or restrictive (Restrict.) temperature before ingestion of opsonized latex beads for 45 min at 37°C. Fluorescence emission was acquired before (a) and after the addition of 10 mM NH4Cl (b). Results are presented as the fluorescence change (ΔF) induced by NH4Cl normalized to the total initial fluorescence of the phagosome (F). Data are means ± SE of at least 40 individual determinations from two separate experiments. Size bar, 5 μm.
Impairment of ubiquitylation also prevented the sorting of the receptor into a multivesicular compartment. Ts20 cells that had been stably transfected with FcγRIIA-GFP were allowed to internalize opsonized latex beads for ∼40 min. As with CHO cells, ts20 cells incubated at the permissive (control) temperature demonstrated a marked increase in phagosomal fluorescence upon addition of ammonia, consistent with the topology of the receptor being inverted, with its cytoplasmic tail in an acidic compartment as a result of receptor sorting to a multivesicular compartment (Figure 7F). In contrast, preincubation for 2 h at the restrictive temperature completely ablated the pH sensitivity of the receptor, consistent with a defect in sorting (Figure 7F, insets a and b) due to impaired ubiquitylation.
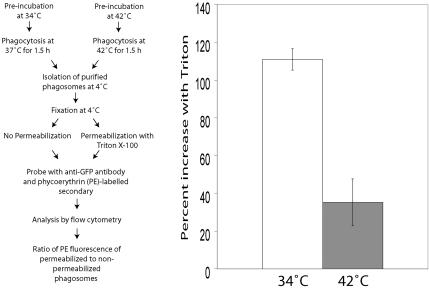
To confirm these results by an alternative approach not involving microscopy, we purified phagosomes (by density centrifugation) from ts20 cells stably expressing FcγRIIA-GFP that had been preincubated at the permissive or restrictive temperature. These experiments were based on the notion that the GFP moiety of the Fc receptor is normally exposed to the cytosolic face of the membrane and should be readily accessible to antibodies added to isolated phagosomes if the receptors maintain their original topology. In contrast, the GFP moiety should become inaccessible to externally added antibodies if receptors are sorted to intraphagosomal vesicles. To distinguish these two topologies, we fixed and probed purified phagosomes with an anti-GFP antibody and phycoerythrin-conjugated secondary antibody, before and after permeabilization of the membrane with a detergent. Exposure of the epitopes was then quantified by flow cytometry. The experimental protocol is detailed schematically in Figure 8 (left panel).
Figure 8.
Measurement of accessibility of the cytosolic tail of FcγRIIA-GFP in isolated phagosomes using flow cytometry. Left panel: flow diagram of the experimental protocol. Ts20 cells stably transfected with FcγRIIA-GFP were incubated at 34 or 42°C for 2 h before ingesting opsonized latex beads. After 1.5 h of phagocytosis, phagosomes were isolated by sucrose density centrifugation, fixed, and either permeabilized with Triton X-100 or left intact. The accessibility of the GFP moiety of the construct, an indicator of the cytosolic tail, was then probed with rabbit anti-GFP and phycoerythrin (PE)-conjugated goat anti-rabbit antibodies. Quantitation was by flow cytometry. Right panel: results are presented as the percentage increase in PE fluorescence induced by permeabilization with Triton. The open bar represents phagosomes from cells at 34°C, and the gray bar represents phagosomes from cells preincubated at 42°C.
As shown in Figure 8 (right panel), phagosomes purified from ts20 cells grown at the permissive temperature exhibited a sizable (≈110%) increase in phycoerythrin (anti-GFP) signal after addition of Triton. This unmasking of a latent population of receptors is consistent with receptor sequestration in intraphagosomal vesicles. In contrast, phagosomes isolated from cells preincubated at the restrictive temperature demonstrated a much smaller increase upon permeabilization (Figure 8, right panel), implying little delivery to an intraphagosomal compartment. The difference between ubiquitination-competent and restricted cells was statistically significant (p < 0.05). These results confirm that during the course of phagosome maturation, the topology of the Fc receptor becomes inverted in a ubiquitin-dependent manner.
Proteasome Inhibition Prevents Sorting of FcγRIIA from the Phagosome to a Multivesicular Compartment
The preceding results implicating ubiquitylation in phagosome maturation were all obtained using ts20 cells transfected with FcγRIIA. This engineered phagocyte system enabled us to attribute the maturation defect to impaired ubiquitylation, because of the specific nature of its temperature-sensitive mutation. It was desirable, however, to confirm these observations by an alternate approach. In several mammalian cell types it has been found that impairment of proteasomal activity results in a time-dependent depletion of the cytosolic pool of mono-ubiquitin (Patnaik et al., 2000; Schubert et al., 2000). We therefore pretreated RAW and CHO cells transfected with FcγRIIA-GFP for 3 h with the proteasome inhibitor MG-132 and allowed them to internalize opsonized latex beads. Pretreatment with the proteasomal inhibitor delayed the redistribution and eventual removal of FcγRIIA-GFP from the phagosomes. As shown in Figure 9 for CHO cells, receptor-associated fluorescence was brighter and persisted longer in phagosomes of MG-132–treated cells compared with untreated controls. After 40 min, the residual fluorescence in the treated cells was 3.7 ± 1.2-fold brighter (p < 0.05) than in untreated controls (Figure 9F). The differences in the pattern of fluorescence induced by MG-132 were also highly significant (p < 0.005; Figure 9E). Although poor transfection efficiency precluded accurate quantitation, we observed the same trend in murine RAW macrophages transfected transiently with FcγRIIA-GFP and pretreated with MG-132 (unpublished data).
Figure 9.
Proteasome inhibition prevents loss of FcγRIIA from the phagosome. CHO cells stably transfected with FcγRIIA-GFP were either left untreated (A) or pretreated with 10 μM MG-132 for 3 h (C) before phagocytosis of opsonized beads for 40 min. The cells were then fixed and the distribution of FcγRIIA-GFP was analyzed by laser confocal microscopy (A and C). Corresponding DIC images appear in B and D. (E and F) Quantitation of results of experiments like those in A–D. The fluorescence pattern of each phagosome was categorized as being continuous around the phagosome, patchy (discontinuous) or absent from the phagosome. The fraction of each type is shown in E for both conditions. (F) The ratio of the fluorescence intensity of phagosomes of cells treated with MG-132 divided by phagosomes of control cells, measured as in Figure 5. Data are means ± SE of three experiments, each counting a minimum of 60 phagosomes. Size bar, 5 μm.
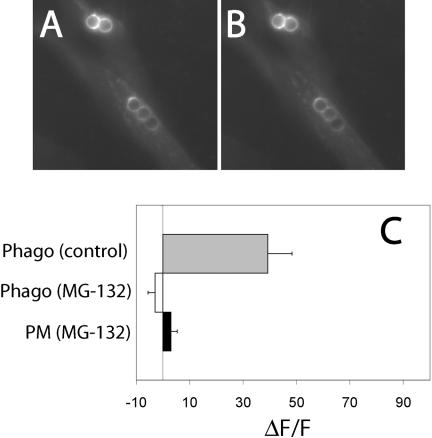
We also assessed whether proteasome inhibition prevented sorting of FcγRIIA-GFP into a multivesicular compartment by measuring the change in receptor-associated fluorescence at or near the phagosome upon addition of ammonia (Figure 10). As before, we found that addition of the weak base to cells stably transfected with FcγRIIA-GFP induced a sizable fluorescence change around phagosomes that had been allowed to mature for ≈ 40 min. In contrast, the fluorescence enhancement, indicative of alkalinization, was ablated by pretreatment with MG-132, implying that the receptors had not been delivered to the acidic lumen of MVB.
Figure 10.
Translocation of the cytosolic tail of FcγRIIA to an acidic compartment is prevented by proteasome inhibitors. CHO cells stably transfected with FcγRIIA-GFP were pretreated with 10 μM MG-132 for 3 h and allowed to ingest opsonized latex beads for 45 min at 37°C. Fluorescence emission was acquired before (A) and after the addition of 10 mM NH4Cl (B). (C) Quantitation of fluorescence change induced by addition of NH4Cl in the immediate vicinity of the phagosome (Phago) and at the plasma membrane (PM) in control and MG-132–treated cells. Results are presented as the fluorescence change (ΔF) induced by NH4Cl normalized to the total initial fluorescence of the phagosome (F). Data are means ± SE of at least 50 individual determinations.
Depletion of Free Ubiquitin Cannot Account for the Effects of Proteasomal Inhibitors
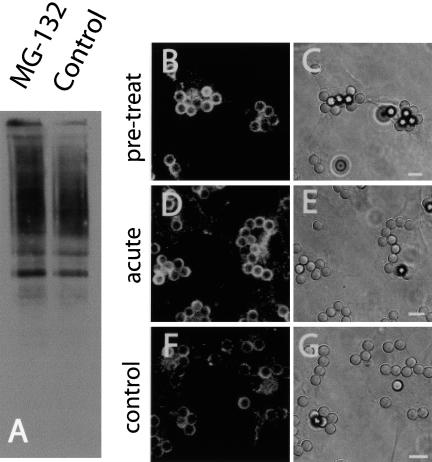
The effects of ubiquitin ligases on membrane traffic are generally thought to be due to the insertion of one or multiple mono-ubiquitin moieties into cargo molecules that are thereby recognized and targeted for rerouting (Hicke, 2001). This role of ubiquitin contrasts with the addition of poly-ubiquitin chains to proteins targeted for proteasomal degradation (Weissman, 2001). It was recently reported, however, that polyubiquitylation and proteasomes may also function in directing receptor endocytosis (van Kerkhof et al., 2001; Longva et al., 2002). It was therefore conceivable that MG-132 might have affected FcγRIIA traffic in macrophages by a mechanism not involving depletion of soluble ubiquitin. To address this possibility, we compared the extent of ubiquitylation in phagosomal membranes purified from control cells or from cells pretreated with the inhibitor. Contrary to our expectation, the abundance of ubiquitylated proteins was greater in phagosomes isolated from MG-132 cells than from paired untreated controls (Figure 11A). This finding may reflect the recruitment to the phagosome of proteins that had been ubiquitylated before depletion of free ubiquitin by the proteasome inhibitor. However, it is also possible that MG-132 did not effectively deplete the pool of ubiquitin. Regardless of the explanation, it is apparent that the effects of the proteasomal inhibitor on FcγRIIA traffic cannot be attributed to reduced ubiquitylation of phagosomal components. It was therefore conceivable that proteasomes might be playing a more direct role in remodelling the phagosomal membrane.
Figure 11.
The effects of proteasome inhibition on fission are not through depletion of cellular free ubiquitin. (A) RAW macrophages were either left untreated (control) or were pretreated with MG-132 for 1.5 h and then allowed to ingest opsonized beads for 12 min at 37°C. Purified phagosomes were next isolated by sucrose density centrifugation as detailed in Materials and Methods. Purified phagosomes were analyzed by immunoblotting using antibodies that recognize both poly- and mono-ubiquitin. (B–G) ts20 cells stably transfected with FcγRIIA-GFP were pretreated as indicated below and then allowed to internalize latex beads for 40 min at 37°C. The cells were fixed and the distribution of receptors was assessed by confocal microscopy (B, D, and F). Corresponding DIC images are shown in C, E, and F. In B and C the cells were pretreated with MG-132 for 3 h prior to phagocytosis. In D–E MG-132 was added 15 min after the onset of phagocytosis. F and G illustrate control cells that were never exposed to MG-132. Images are representative of three separate experiments. Size bars, 5 μm.
To test this notion, we applied MG-132 to the cells 15 min after completion of phagocytosis and monitored the fate of FcγRIIA. Elimination of the prolonged preincubation period ensured that, although proteasomal activity would be acutely inhibited, the free ubiquitin pool would remain largely unaffected. As shown in Figure 11, the segregation and elimination of receptors from the phagosome was markedly inhibited by the short incubation (cf. panels D and F in Figure 11), to an extent that was indistinguishable from that observed with long MG-132 preincubations. Several observations suggest that this effect is a specific consequence of proteasomal inhibition and not due to nonspecific damage. First, addition of a chemically unrelated proteasome inhibitor, clasto-lactacystin β-lactone (20 μM for 1 h) had effects similar to those of MG-132 (unpublished data). Second, neither MG-132 nor clasto-lactacystin β-lactone impaired the ability of the cells to engulf the opsonized particles. Third, as described below, MG-132 had no effect on fusion of phagosomes with lysosomes.
Proteasome Inhibition Does Not Impair Fusion of Phagosomes with Lysosomes
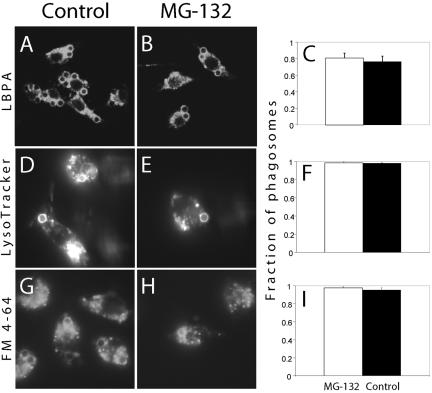
We next assessed whether normal receptor fission and MVB formation were essential for fusion of endosomes and lysosomes with the phagosome. Fusion with late endosomes was assessed by measuring the acquisition of LBPA, while lysosome fusion was quantified by preloading this compartment with a fluid-phase marker followed by an extended (2.5 h) chase period or using LysoTracker Red. RAW macrophages were either pretreated with MG-132 or left untreated, induced to ingest opsonized beads and then allowed to undergo maturation for varying periods of time. For merger with late endosomes, the phagosomes were allowed to mature for 40 min and immunostained for LBPA. Lysosomal fusion was assessed >50 min after phagocytosis. As illustrated in Figure 12, pretreatment with MG-132 did not significantly alter fusion of phagosomes with either late endosomes (Figure 12, A–C) or with lysosomes (Figure 12, D–I). These observations suggest that phagosomal fission and fusion events are controlled separately and imply also that the effects of the proteasomal inhibitor on FcγRIIA processing are not due to generalized deleterious effects of the drug.
Figure 12.
Proteasome inhibition has no effect on fusion of phagosomes with MVB or lysosomes. RAW macrophages were either left untreated (A, D, and G) or were pretreated with 10 μM MG-132 for 1.5 h (B, E, and H) and allowed to ingest opsonized beads for 45–60 min at 37°C. Cells were then fixed and permeabilized before immunostaining for LBPA (A and B) or incubated with LysoTracker Red for 5 min (D and E). In G and H, RAW cells were pulsed with FM 4-64 for 1 h and then chased for an additional 1 h and left otherwise untreated or pretreated with MG-132 as above. Subsequently, cells were allowed to ingest opsonized beads for 60 min at 37°C. (C, F, and I) Summaries of quantifications of the fraction of phagosomes in MG-132 pretreated (light bars) and control cells (dark bars) that were positively stained with LBPA antibodies, LysoTracker Red, and FM4-64, respectively. Data are means of three experiments, each counting at least 50 phagosomes.
DISCUSSION
Despite undergoing multiple rounds of fusion with components of the endocytic pathway and possibly also the secretory pathway, it is remarkable that maturing phagosomes maintain a fairly constant size. This fact, and the observation that certain plasma membrane receptors internalized during phagocytosis are subsequently detected on the cell surface, attests to the existence of one or more mechanisms of membrane fission from the phagosome. The importance of fission events is highlighted by the finding that antigenic peptides derived from pathogens ingested by phagocytosis are subsequently presented on the cell surface as complexes with MHC-II molecules (Ramachandra et al., 1999).
Remarkably little is known about the molecular mechanisms underlying phagosomal fission. Recent work has implicated microfilaments and microtubules in recycling from the phagosome (Damiani and Colombo, 2003). To date, only a minor role for the coatomer complex COPI has been described; clearance of transferrin receptors from maturing phagosomes was modestly reduced in cells lacking the ε-subunit of the COP complex, whereas clearance of FcγRIIA receptors was unaffected (Botelho et al., 2000). In another study, no role for clathrin-dependent vesiculation could be demonstrated using dominant-negative alleles of dynamin (Tse et al., 2003).
Sorting of the epidermal growth factor (EGF) receptors and other tyrosine kinase receptors into MVB has been reported by others and is thought to involve a ubiquitin-dependent process (Katzmann et al., 2002; Longva et al., 2002). The finding that ubiquitylation is triggered by FcγR cross-linking (Booth et al., 2002) and that abundant ubiquitylation was detected on phagosomes suggested that a similar mechanism may apply to tyrosine kinase-associated receptors and that structures related to MVB may be generated by phagosomes. Accordingly, we found that normal FcγR clearance from phagosomes requires ubiquitylation. Remarkably, our data suggest that not only ubiquitin, but also proteasomal function is required for normal receptor elimination from phagosomes. Recruitment of proteasomes to phagosomes was reported recently and attributed a role in antigen processing leading to cross-presentation by MHC class I (Houde et al., 2003). It is conceivable that proteasomes may play additional roles in phagosomal function, including the processing of phagosomal membrane proteins. Proteasomal proteins were very recently found to be ligands of active Rab7 and this GTPase is characteristically present on the membrane of late phagosomes and phagolysosomes (Dong et al., 2004). It will therefore be of interest to establish whether Rab7 function is required for FcγR clearance from phagosomes.
Proteasomes could eliminate FcγR simply by degradation of their ubiquitylated cytosolic tails. However, it is unclear in this instance how the transmembrane and luminal segments of the receptor would be disposed. Indeed, our data indicate that clearance of FcγRIIA from the maturing phagosome occurs, at least in part, by sorting of the receptors into MVB. The delivery of receptors to MVB was inferred from the finding that their cytosolic domain becomes exposed to an acidic milieu, compatible with the lumen of an endomembrane and different from the cytosol. As corroboration, we were able to directly document delivery of the cytosolic moiety of GFP-tagged receptors into an intraphagosomal compartment by flow cytometry of purified phagosomes. Indeed, using TEM we observed multivesicular structures in the immediate vicinity of phagosomes, as well as membrane vesicles in the phagosomal lumen. These vesicles increased substantially in abundance during phagosome maturation. It is unclear whether the vesicles seen by TEM are formed by invagination of the phagosomal membrane or are the result of fusion with existing MVB, so it is not currently possible to define precisely the direction of fission. However, the pH sensitivity of fluorescent spots closely associated with the phagosome is consistent with the occurrence of inward budding. Fluorescent vesicles that are seemingly unconnected to the phagosome are also often seen in its vicinity, and their sensitivity to permeant weak bases indicates that these are receptor-bearing MVB. In cells transfected with conventional GFP it is impossible to discount the possibility that such MVB are derived from endocytic events at the plasma membrane, as opposed to the phagosome. However, we were able to observe fluorescent vesicles detach from phagosomes where receptor fluorescence was photoactivated in situ. This implies that at least a fraction of the phagosomal receptors are cleared from the phagosomes by outward fission. The resulting structures may be multivesicular, as shown for diagrammatic convenience in Figure 4 (transition 3a to 4a), or may be initially normally oriented single vesicles that proceed to fuse with late endosomes/lysosomes, undergoing inward budding subsequently.
Although proteins that are directed to the inner vesicles of MVB are usually degraded, it has recently been shown that at least some of them are able to recycle back to the limiting membrane of the endosome (Gruenberg and Stenmark, 2004). This is unlikely to be the main fate of Fc receptors because it is known that they are degraded in lysosomes after activation by cross-linking (Mellman and Plutner, 1984; Ukkonen et al., 1986). Sorting of the internalized receptor into a MVB is therefore likely to be an intermediate step leading to lysosomal degradation. Degradation could occur within the lumen of the phago-lysosome for those receptors that bud inward, or after fusion with lysosomes for those that segregate with cytoplasmic vesicles that pinch off the phagosomal membrane. The relative contributions of these two components remains to be defined.
Remarkably, interference with fission from the phagosome had little effect on ongoing fusion events. Treatment with proteasome inhibitors had no discernible impact on the formation of phagolysosomes, the ultimate stage of phagosome maturation. This finding is in agreement with the earlier report that inhibition of ubiquitylation in ts20 cells had no effect on LAMP1 acquisition by maturing phagosomes (Booth et al., 2002). Thus, although fusion and fission of membranes to and from the phagosome seem to be functionally coordinated, they are nevertheless independent processes with separate molecular mechanisms.
In endosomes, sorting of ubiquitylated receptors involves the recruitment of a panoply of proteins in a process that requires phosphatidylinositol 3′-phosphate (PI(3)P). Sorting is initiated by Vps27 (Hrs), a critical adaptor that is believed to be directed to the membrane by its FYVE domain, which recognizes PI(3)P. Hrs also interacts directly with ubiquitylated cargo by means of its UIM motif and the complex serves as a docking site for the ESCRT-I system (Raiborg et al., 2003). A similar process appears to be at work during phagosomal maturation.
Evidence already exists that PI(3)P (Fratti et al., 2001; Vieira et al., 2001) and Hrs (Vieira et al., 2004) are recruited to the phagosomal membrane and play a critical role in its remodelling. The present observations that ubiquitylated proteins are present in phagosomes and are required for receptor clearance fit well with existing model of MVB formation. What remains unclear is what role proteasomes may play in this sequence. One possibility is that a negative regulator of budding needs to be degraded by the proteasome for MVB formation to proceed. Failure to degrade this putative inhibitor would impede fission (van Kerkhof et al., 2001). Alternatively, proteasomes may “trim” polyubiquitylated receptors. Receptors that are mono-ubiquitylated at the membrane yet prevented from undergoing endocytosis by being attached to the ligands on the particle are likely to undergo additional rounds of ubiquitylation. Proteasomes near the surface of the phagosome may regenerate mono-ubiquitylated species that are recognized by the targeting machinery of the endocytic pathway.
In summary, we have identified a mechanism whereby receptors, and likely also other molecules are cleared from the limiting membrane of the phagosome. This process, which involves ubiquitin and proteasomes, may contribute to termination of receptor signaling. We speculate that ubiquitin and/or proteasomes may be similarly required for MHC II-dependent presentation of antigens engulfed by phagocytosis. This notion needs to be tested experimentally.
Acknowledgments
We thank Dr. A. Jankowski, who performed some preliminary experiments, and Mr. R. Temkin for technical assistance with electron microscopy. This work was supported by grants from the Canadian Institutes of Health Research (CIHR), the Canadian Arthritis Society, and the National Institutes of Health. W.L.L. is the recipient of a postdoctoral fellowship award from the CIHR in partnership with the Canadian Lung Association, postgraduate awards from the Faculty of Medicine (University of Toronto), and a Medical Scientist Training Fellowship from the McLaughlin Centre for Molecular Medicine. S.G. is the current holder of the Pitblado Chair in Cell Biology and is cross-appointed to the Department of Biochemistry, University of Toronto.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-06-0464) on February 9, 2005.
References
- Babst, M., Odorizzi, G., Estepa, E. J., and Emr, S. D. (2000). Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic 1, 248-258. [DOI] [PubMed] [Google Scholar]
- Booth, J. W., Kim, M. K., Jankowski, A., Schreiber, A. D., and Grinstein, S. (2002). Contrasting requirements for ubiquitylation during Fc receptor-mediated endocytosis and phagocytosis. EMBO J. 21, 251-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botelho, R. J., Hackam, D. J., Schreiber, A. D., and Grinstein, S. (2000). Role of COPI in phagosome maturation. J. Biol. Chem. 275, 15717-15727. [DOI] [PubMed] [Google Scholar]
- Damiani, M. T., and Colombo, M. I. (2003). Microfilaments and microtubules regulate recycling from phagosomes. Exp. Cell Res. 289, 152-161. [DOI] [PubMed] [Google Scholar]
- Desjardins, M., Huber, L. A., Parton, R. G., and Griffiths, G. (1994). Biogenesis of phagolysosomes proceeds through a sequential series of interactions with the endocytic apparatus. J. Cell Biol. 124, 677-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins, M., Nzala, N. N., Corsini, R., and Rondeau, C. (1997). Maturation of phagosomes is accompanied by changes in their fusion properties and size-selective acquisition of solute materials from endosomes. J. Cell Sci. 110(Pt 18), 2303-2314. [DOI] [PubMed] [Google Scholar]
- Dong, J., Chen, W., Welford, A., and Wandinger-Ness, A. (2004). The proteasome alpha-subunit XAPC7 interacts specifically with Rab7 and late endosomes. J. Biol. Chem. 279, 21334-21342. [DOI] [PubMed] [Google Scholar]
- Downey, G. P., Botelho, R. J., Butler, J. R., Moltyaner, Y., Chien, P., Schreiber, A. D., and Grinstein, S. (1999). Phagosomal maturation, acidification, and inhibition of bacterial growth in nonphagocytic cells transfected with FcgammaRIIA receptors. J. Biol. Chem. 274, 28436-28444. [DOI] [PubMed] [Google Scholar]
- Drose, S., and Altendorf, K. (1997). Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases. J. Exp. Biol. 200(Pt 1), 1-8. [DOI] [PubMed] [Google Scholar]
- Fratti, R. A., Backer, J. M., Gruenberg, J., Corvera, S., and Deretic, V. (2001). Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J. Cell Biol. 154, 631-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimuro, M., Sawada, H., and Yokosawa, H. (1994). Production and characterization of monoclonal antibodies specific to multi-ubiquitin chains of polyubiquitinated proteins. FEBS Lett. 349, 173-180. [DOI] [PubMed] [Google Scholar]
- Gessner, J. E., Heiken, H., Tamm, A., and Schmidt, R. E. (1998). The IgG Fc receptor family. Ann. Hematol. 76, 231-248. [DOI] [PubMed] [Google Scholar]
- Gotthardt, D., Warnatz, H. J., Henschel, O., Bruckert, F., Schleicher, M., and Soldati, T. (2002). High-resolution dissection of phagosome maturation reveals distinct membrane trafficking phases. Mol. Biol. Cell 13, 3508-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg, J., and Stenmark, H. (2004). The biogenesis of multivesicular endosomes. Nat. Rev. Mol. Cell. Biol. 5, 317-323. [DOI] [PubMed] [Google Scholar]
- Haglund, K., Sigismund, S., Polo, S., Szymkiewicz, I., Di Fiore, P. P., and Dikic, I. (2003). Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat. Cell Biol. 5, 461-466. [DOI] [PubMed] [Google Scholar]
- Handley-Gearhart, P. M., Trausch-Azar, J. S., Ciechanover, A., and Schwartz, A. L. (1994). Rescue of the complex temperature-sensitive phenotype of CHO E36ts20 cells by expression of the human ubiquitin-activating enzyme cDNA. Biochem. J. 304(Pt 3), 1015-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicke, L. (2001). Protein regulation by monoubiquitin. Nat. Rev. Mol. Cell. Biol. 2, 195-201. [DOI] [PubMed] [Google Scholar]
- Houde, M., Bertholet, S., Gagnon, E., Brunet, S., Goyette, G., Laplante, A., Princiotta, M. F., Thibault, P., Sacks, D., and Desjardins, M. (2003). Phagosomes are competent organelles for antigen cross-presentation. Nature 425, 402-406. [DOI] [PubMed] [Google Scholar]
- Indik, Z. K., Park, J. G., Hunter, S., and Schreiber, A. D. (1995). The molecular dissection of Fc gamma receptor mediated phagocytosis. Blood 86, 4389-4399. [PubMed] [Google Scholar]
- Katzmann, D. J., Babst, M., and Emr, S. D. (2001). Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 106, 145-155. [DOI] [PubMed] [Google Scholar]
- Katzmann, D. J., Odorizzi, G., and Emr, S. D. (2002). Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell. Biol. 3, 893-905. [DOI] [PubMed] [Google Scholar]
- Longva, K. E., Blystad, F. D., Stang, E., Larsen, A. M., Johannessen, L. E., and Madshus, I. H. (2002). Ubiquitination and proteasomal activity is required for transport of the EGF receptor to inner membranes of multivesicular bodies. J. Cell Biol. 156, 843-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman, I., and Plutner, H. (1984). Internalization and degradation of macrophage Fc receptors bound to polyvalent immune complexes. J. Cell Biol. 98, 1170-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patnaik, A., Chau, V., and Wills, J. W. (2000). Ubiquitin is part of the retrovirus budding machinery. Proc. Natl. Acad. Sci. USA 97, 13069-13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, G. H., and Lippincott-Schwartz, J. (2002). A photoactivatable GFP for selective photolabeling of proteins and cells. Science 297, 1873-1877. [DOI] [PubMed] [Google Scholar]
- Pitt, A., Mayorga, L. S., Stahl, P. D., and Schwartz, A. L. (1992). Alterations in the protein composition of maturing phagosomes. J. Clin. Invest. 90, 1978-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiborg, C., Rusten, T. E., and Stenmark, H. (2003). Protein sorting into multivesicular endosomes. Curr. Opin. Cell Biol. 15, 446-455. [DOI] [PubMed] [Google Scholar]
- Ramachandra, L., Song, R., and Harding, C. V. (1999). Phagosomes are fully competent antigen-processing organelles that mediate the formation of peptide:class II MHC complexes. J. Immunol. 162, 3263-3272. [PubMed] [Google Scholar]
- Rosenberger, C. M., and Finlay, B. B. (2003). Phagocyte sabotage: disruption of macrophage signalling by bacterial pathogens. Nat. Rev. Mol. Cell. Biol. 4, 385-396. [DOI] [PubMed] [Google Scholar]
- Schubert, U., Ott, D. E., Chertova, E. N., Welker, R., Tessmer, U., Princiotta, M. F., Bennink, J. R., Krausslich, H. G., and Yewdell, J. W. (2000). Proteasome inhibition interferes with gag polyprotein processing, release, and maturation of HIV-1 and HIV-2. Proc. Natl. Acad. Sci. USA 97, 13057-13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse, S.M.L., Furuya, W., Gold, E. Schreiber, A. D., Sandvig, K., Inman, R. D., Grinstein, S. (2003). Differential role of actin, clathrin, and dynamin in Fcgamma receptor-mediated endocytosis and phagocytosis. J. Biol. Chem. 278, 3331-3338. [DOI] [PubMed] [Google Scholar]
- Ukkonen, P., Lewis, V., Marsh, M., Helenius, A., and Mellman, I. (1986). Transport of macrophage Fc receptors and Fc receptor-bound ligands to lysosomes. J. Exp. Med. 163, 952-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kerkhof, P., Alves dos Santos, C. M., Sachse, M., Klumperman, J., Bu, G., and Strous, G. J. (2001). Proteasome inhibitors block a late step in lysosomal transport of selected membrane but not soluble proteins. Mol. Biol. Cell 12, 2556-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira, O. V., Botelho, R. J., Rameh, L., Brachmann, S. M., Matsuo, T., Davidson, H. W., Schreiber, A., Backer, J. M., Cantley, L. C., and Grinstein, S. (2001). Distinct roles of class I and class III phosphatidylinositol 3-kinases in phagosome formation and maturation. J. Cell Biol. 155, 19-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira, O. V., Harrison, R. E., Scott, C. C., Stenmark, H., Alexander, D., Liu, J., Gruenberg, J., Schreiber, A. D., and Grinstein, S. (2004). Acquisition of Hrs, an essential component of phagosomal maturation, is impaired by mycobacteria. Mol. Cell. Biol. 24, 4593-4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman, A. M. (2001). Themes and variations on ubiquitylation. Nat. Rev. Mol. Cell. Biol. 2, 169-178. [DOI] [PubMed] [Google Scholar]