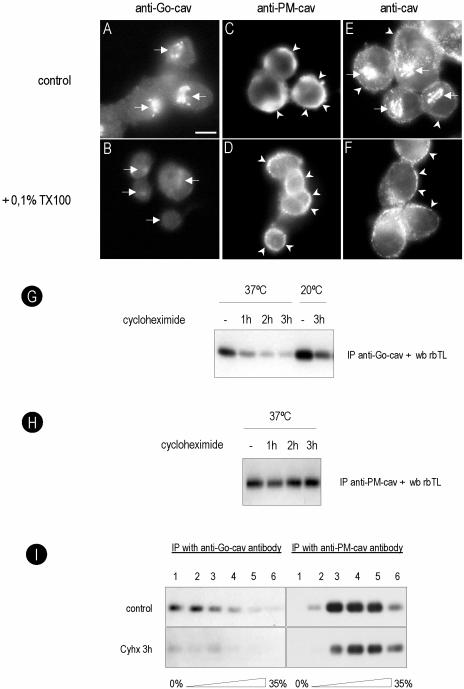
Figure 4.
Golgi caveolin is detergent soluble and forms low-molecular-weight oligomers. BHK cells were extracted for 2 min at 4°C with a buffer containing 0.1% TX-100, fixed in PFA, and caveolin was detected with an anti-Go-cav or an anti-PM-cav antibody. The Golgi pool of caveolin was completely extracted by the detergent (compare A and B or E and F), but the PM pool was largely unaffected (compare C and D or E and F). In G, BHK cells were treated for 1, 2, or 3 h with Cyhx at 37 or 20°C, extracted for 2 min at 4°C with a buffer containing 0.1% TX-100, and the soluble fraction was immunoprecipitated with anti-Go-cav antibody. The amount of caveolin decreased progressively in response to Cyhx, and little protein was detected after 3 h. When the cells were incubated at 20°C, the amount of caveolin immunoprecipitated increased. At 20°C, no effect was observed in response to Cyhx consistent with retention in the Golgi complex. No changes in response to Cyhx were observed with an anti-PM-cav antibody (H). (I) Control cells or cells treated for 3 h with Cyhx were extracted with 0.1% TX-100, and the different molecular weight oligomers of caveolin were fractionated using a sucrose gradient (see Materials and Methods for details). Fractions of the gradient were immunoprecipitated using anti-Go-cav or anti-PM-cav. The anti-Go-cav antibody recognized predominantly low-molecular-weight oligomers of the protein (fractions 1 and 2 of the gradient), but the anti-PM-cav antibody exclusively recognized the high-molecular-weight caveolin complexes (fractions 3–5). In contrast to the PM pool of the protein, Golgi caveolin was sensitive to Cyhx. Bar, 5 μm.