Figure 2.
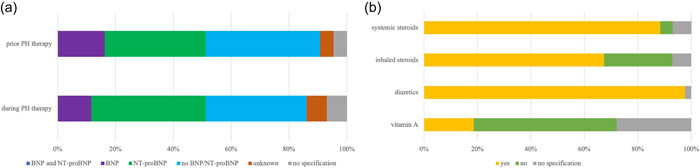
Diagnostic and therapy in premature infants with developing (at risk) or established bronchopulmonary dysplasia (BPD)‐associated pulmonary hypertension (PH). (a) The use of biomarkers BNP and NT‐proBNP in preterm infants with developing (at risk) or established BPD‐associated PH before initiation of therapy in contrast monitoring PH during therapy. None of the participants reported using the combination of BNP and NT‐proBNP before or during therapy. Before initiation of targeted PH therapy to monitor disease progression, individual markers were used in 35% (15/43) (NT‐proBNP), 16% (7/43) (BNP), and 40% (17/43) of participants reported not determining either BNP or NT‐proBNP before initiation of therapy. A total of 40% (17/43) used NT‐proBNP and 12% (5/43) used BNP in infants with developing (at risk) or established BPD‐associated PH during therapy, in contrast to 35% (15/43) of participants who used neither BNP nor NT‐proBNP to monitor high‐risk patients during therapy. (b) Prescription of therapy in preterm infants with developing (at risk) or established BPD‐associated PH. For infants with developing or established BPD‐associated PH before discharge, 98% (42/43) of participants reported the prescription diuretics in addition to systemic steroids (88%, 38/43), whereas prescription of inhalative steroids were reported by 67% (29/43). The recommendation of vitamin A prescription was reported by only 19% (8/43). Most participants (54%, 23/43) documented to not use vitamin A in infants with developing or established BPD‐associated PH, whereas inhalative and systemic steroids were reported by only 26% (11/43) and 5% (2/43). The data are provided as the percentage of the total number of participants (43) and items (73) in the final analysis as well as by total n‐numbers.
