Scheme 1. Synthesis of Fluorination Precursor 11.
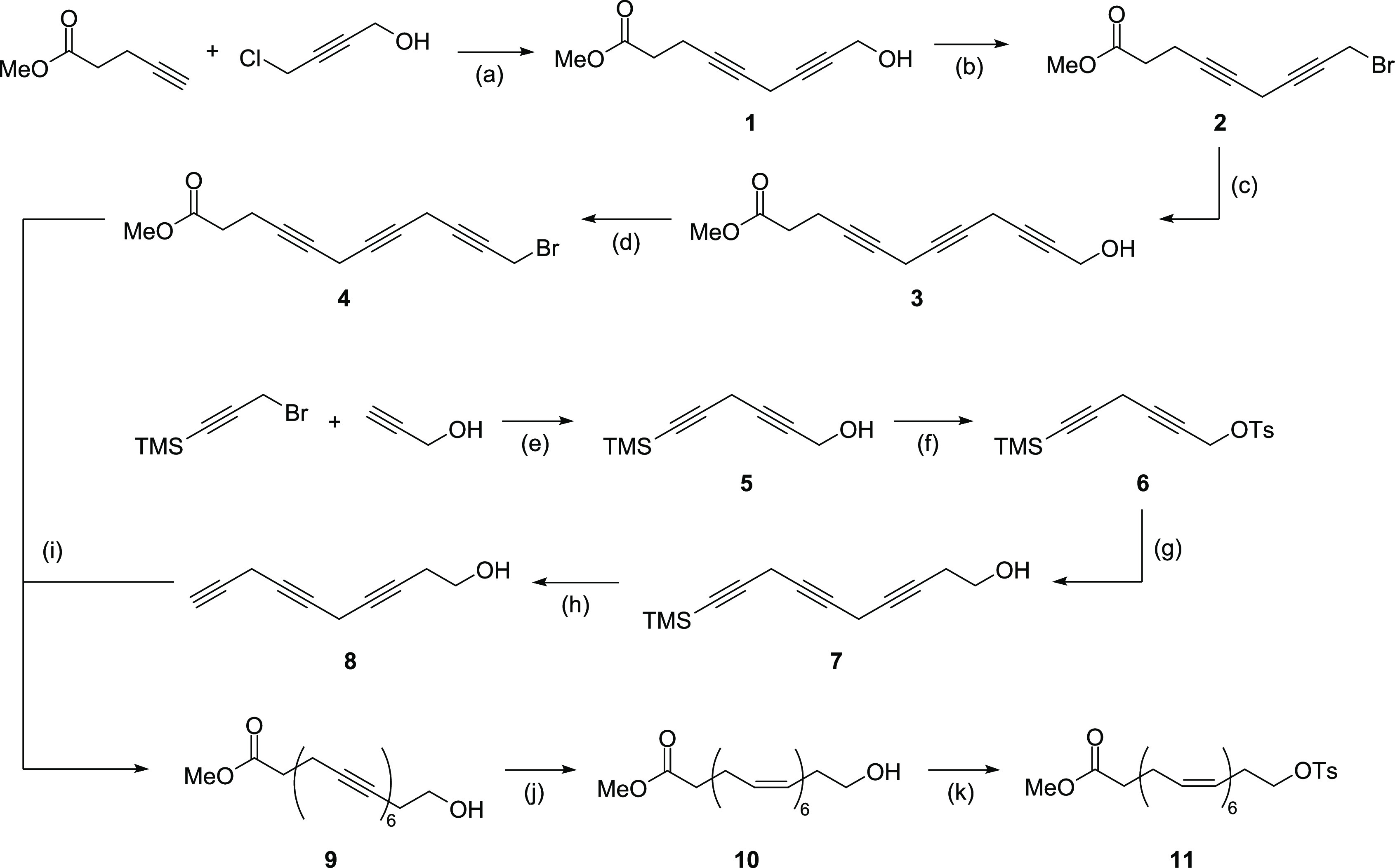
(a) CuI, NaI, Cs2CO3, DMF, rt, 83% yield; (b) CBr4, PPh3, DCM, 0 °C, 80% yield; (c) propargyl alcohol, CuI, NaI, Cs2CO3, DMF, rt, 65% yield; (d) CBr4, PPh3, DCM, 0 °C, 61% yield; (e) CuI, NaI, Cs2CO3, DMF, rt, 94% yield; (f) Ts2O, pyridine, DCM, rt, 60% yield; (g) 3-butyn-1-ol, CuI, NaI, Cs2CO3, DMF, rt, 48% yield; (h) TBAF, acetic acid, THF, 0 °C, 84% yield; (i) CuI, NaI, Cs2CO3, DMF, rt, 41% yield; (j) H2 (1 atm), Lindlar’s catalyst, 4:2:2:1 (v/v) 2-methyl-2-butene/EtOAc/methanol/pyridine, rt; (k) TsCl, Et3N, DMAP, DCM, 0 °C to rt, 13% total yield over two steps from compound 9.
