Abstract
Objective
Oropharyngeal squamous cell carcinoma (OPSCC) recurrence is almost universally fatal. Development of effective therapeutic options requires an improved understanding of recurrent OPSCC biology.
Methods
We analyzed paired primary-recurrent OPSCC from Veterans treated at the Michael E. DeBakey Veterans Affairs Medical Center between 2000 and 2020 who received curative intent radiation-based treatment (with or without chemotherapy). Patient tumors were analyzed using standard immunohistochemistry and automated imaging of infiltrating lymphocytes and multinucleated tumor cells coupled to machine learning algorithms.
Results
Primary and recurrent tumors demonstrated high concordance via p16 and p53 immunohistochemistry, with comparable levels of multinucleation. In contrast, recurrent tumors demonstrated significantly higher levels of CD8+ tumor infiltrating lymphocytes (p<0.05) and higher levels of PD-L1 expression (p<0.05).
Conclusion
Exposure to chemo-radiation and recurrence following treatment preserves critical features of intrinsic tumor biology and the tumor immune microenvironment suggesting that novel treatment regimens may be as effective in the salvage setting as in the definitive intent setting.
Keywords: Oropharyngeal cancer, Multinucleation, Tumor infiltrating lymphocytes, Recurrence
Introduction
Infection with high-risk subtypes of human papillomavirus (HPV) has driven an epidemic rise in the incidence of oropharyngeal squamous cell carcinoma (OPSCC) [1–5], a disease formerly associated exclusively with heavy tobacco exposure [6, 7]. Although a majority of new HPV-associated OPSCC diagnoses occur in patients with either no history of tobacco exposure or limited past exposure, select patient populations including Veterans and indigent patients maintain high rates of tobacco exposure and concomitantly high rates of tobacco associated cancers [7–11]. In the context of OPSCC, tobacco exposure that exceeds 10 pack years cumulatively prior to diagnosis generates an “intermediate-risk” phenotype that is associated with a nearly 25% reduction in survival across both prospective and retrospective cohorts [12–15]. This phenotype, exacerbated by extensive tobacco exposure exceeding 30 pack years, has been shown by us to drastically reduce survival in Veteran cohorts despite equivalent deployment of combined multi-modality treatment (chemo-radiation) [7, 8].
We previously robustly linked survival and chemo-radiation response in OPSCC to a combination of intrinsic tumor biological factors, namely the presence of multinucleated cells and geo-spatial organization of infiltrating lymphocytes [8, 16–19]. Although these factors have been linked by our group with survival in low-risk and intermediate-risk HPV-associated and high-risk HPV-independent OPSCC, it remains unclear whether these biological features are impacted by completion of curative intent treatment or whether they are retained post-treatment.
Intermediate- and high-risk OPSCC demonstrate high rates of in-field or locoregional recurrence consistent with other HPV-independent disease sites such as oral cavity [7, 20]. The viability of salvage treatment whether it consists of surgery, re-irradiation, or systemic treatment with chemotherapy with or without immune checkpoint inhibitors is driven by relative therapeutic index ( anti-tumor effectiveness / excess toxicity). Anti-tumor effectiveness of OPSCC treatment has now been linked by us and others to intrinsic tumor biology and anti-tumor immunity [7, 8, 16–18, 21]. In the current manuscript we sought to determine whether definitive, curative intent chemo-radiation generated a deleterious shift in tumor biology and immunity in the context of OPSCC enriched for intermediate- and high-risk disease that could negatively impact the effectiveness of salvage treatment.
Materials and Methods
Clinical Dataset
Following approval from Baylor College of Medicine and the Michael E. DeBakey Veteran’s Administration (MEDVAMC) Institutional Review Board, we reviewed the records of Veterans with oropharyngeal squamous cell carcinoma (OPSCC) between January 1, 2000 and January 1, 2020. Data collection and analysis was performed in a manner consistent with existing standards for clinical research (Declaration of Helsinki, US Federal Policy for the Protection of Human Subjects). Inclusion criteria included: 1) primary OPSCC, 2) tissue diagnosis at the MEDVAMC, 3) treatment delivery at the MEDVAMC, 4) at least 2 years of post-treatment surveillance at the MEDVAMC and 5) documented recurrence/metastasis at the MEDVAMC. All patients were required to have tissue available for diagnostic assessment and performance of research studies. Since the research testing required tumor tissue with intact morphology, patients that underwent diagnosis of primary tumor or recurrent disease via fine needle aspiration were excluded.
From each patient, we generated a tumor pair, consisting of tissue from the primary, pre-treatment tumor and the recurrent post-treatment tumor. Due to limitations in the amount of tissue available (secondary to small biopsy sizes for several patients), each individual datapoint (e.g., p16 status) was not available for every single patient and every single primary-recurrent tumor pair. As such, throughout the Results section, we refer to the number of tumors for which that particular datapoint (e.g., p16 status) is available as the denominator for a given positive or negative rate.
Due to the retrospective nature of the study and the minimal risk profile, waiver of consent was obtained from the IRB prior to research study commencement. Exclusion criteria included treatment at an outside institution and lack of recurrent disease. Demographic information including age, gender, race, smoking history was collected and analyzed. Clinical and pathologic features were collected using the American Joint Commission on Cancer (Staging Manual 7th Edition) staging system. Given the high preponderance of high- and intermediate-risk OPSCC, the 7th Edition provides a more nuanced and clinically relevant staging summary of OPSCC compared to the newer 8th Edition, which drastically de-escalated N-classification regardless of relative tumor risk [22]. Results of diagnostic results and biopsies, treatments delivered and the associated dates were recorded.
Quantitative Immunohistochemistry
We have previously described our quantitative assessment of p16 immunohistochemistry along with quantitative measurements of infiltrative CD3+ and CD8+ lymphocytes [19]. Briefly, formalin-fixed paraffin-embedded (FFPE) biopsy tissue blocks were retrieved from the archive maintained at the MEDVAMC Department of Pathology. Slides were deparaffinized and rehydrated prior to the blocking of endogenous peroxide activity. Immuno-histochemical (IHC) stains were performed on an automated tissue-staining system using the Bond Polymer Refine Detection (Leica Biosystems, Buffalo Grove, IL) using antibodies for CD3: LN10, CD8: 4B11, p53: DO-7, PDL1: 73-10 (Leica Biosystems, Richmond IL), and p16 E6H4 (Ventana, Tucson, Arizona). Representative images of IHC were captured using the Vectra3 scanner using the CRI multispectral camera using a 20x UplanSApo objective (0.75 aperture, 0.5um/pixel) mounted on an Olympus microscope. (Akoya Biosciences, Menlo Park, CA) and analyzed using InForm 2.4 software (Akoya). RNA Scope images were captured using a Nikon Eclipse scanning microscope with a Nikon DS-Ri1 camera [40X Plan Apo objective (0.75 aperture, 0.23um/pixel)]. The percent of positively stained features was calculated as the number of positive cells divided by the total number of cells in the tumor segmented areas. p53 positive cells were characterized by strong nuclear staining by IHC in the tumor areas of the tissue (Figure 1). An aberrant p53 phenotype was defined by the absence of staining (null; threshold of 5%) or >80% of positive cells (mutant) in the tumor [23, 24]. Assignment of p16 status (positive vs negative) was defined using the most recently published guidelines from Lewis Jr. et al. [25] of >70% strong nuclear and cytoplasmic staining as identified by the primary pathologist involved in the individual cases. Combined positive score (CPS) was calculated for PD-L1 as follows: PD-L1 positive cells (tumor cells, lymphocytes & macrophages)/total number of viable cells (Figure 2). CPS ≥1 was scored as positive for PD-L1. High-risk HPV RNA in situ hybridization was performed using the HPV-HR18 cocktail (Advanced Cell Diagnostics, Newark, CA) using standard protocols for 2.5 HD Assay-Brown. This assay hybridizes to the E6/E7 mRNa for the following high-risk HPV types: 16,18,26,31,33,35,39,45,51,52,53,56,58,59,66,68,73 and 82. HPV16 mRNA positive OPSCC was used as a control. DAB positivity of the HPV-HR18 probe in any part of the tumor area were scored as positive for HPV.
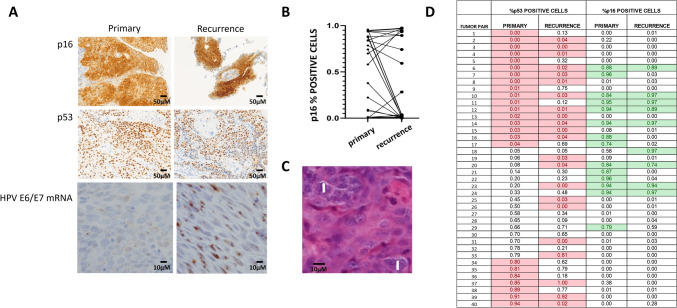
Fig. 1.
A Representative staining of primary and recurrent tumors for p16, p53 and high risk HPV E6/E7 mRNA. B Changes in p16 status between primary and recurrent tumors. C Examples of multinucleated cells denoted by white vertical bars within a primary tumor. D Shifts in p53 and p16 positivity expressed as fraction of cells noted to be positive in each primary or recurrent tumor sample from 40 tumor pairs. Red highlighted boxes denote aberrant p53 staining; green highlighted boxes denote p16 positivity in excess of 0.70 (scale bars represent 50µm and 10 µm, respectively)
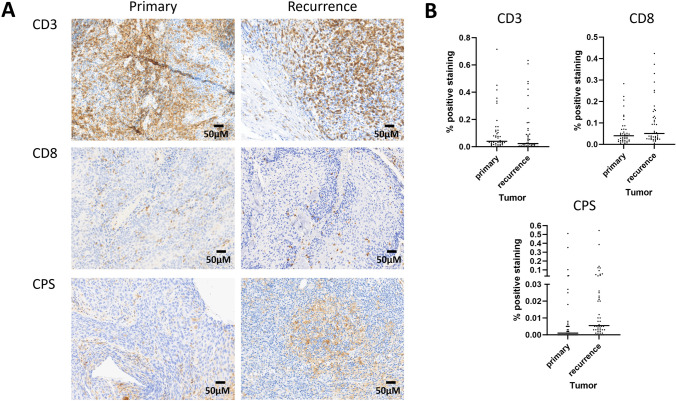
Fig. 2.
A Representative staining of primary and recurrent tumors for CD3, CD8, and PDL1. B Distribution of immune infiltration and PDL1 positive cells across primary and recurrent tumors (scale bars represents 50µm)
Computerized Analysis of Tissue Specimens
Hematoxylin and eosin (H&E) stained slides were digitized by scanning using Ventana iScan HT at 40x resolution (0.25 μm/pixel resolution). HistoQC [26] was used to identify cases inappropriate for the study due to the presence of large blurry areas, obstructive dotting pen markings, or subcoverslip bubbles. Multinucleation frequency was calculated using a previously described algorithm [17], which generated a multinucleation index (MuNI) characterizing the density of events in epithelial regions. More specifically, MuNI scores reflect the frequency of multinucleated cells (Figure 1C) as a function of the total number of epithelial cells in the individual tumor. Tumor infiltrating lymphocyte (TIL) frequency and intra-tumoral distribution was quantified using a validated machine learning algorithm (OP-TIL). Briefly, OP-TIL score is an aggregate measure (inclusive of density, architecture and cell co-localization) of intra-tumoral TIL number and the spatial interactions between TILs and non-TIL intra-tumoral cells. The algorithm has been developed, trained and validated on OPSCC [18]. The MuNI and OP-TIL risk scores were computed as continuous variables for each patient and a cutoff was then used to stratify patients into low- and high-risk categories as previously described in [17, 18].
Results
Patient and Disease Characteristics
We reviewed data from a total of 56 male patients with a diagnosis of OPSCC, with a mean age of 59.5, a heavy tobacco exposure history (Table 1; median pack year history = 40), and a high rate of nodal metastasis (45/56, 80%) at time of presentation. Approximately 50% (28/56) of patients experienced local recurrence exclusively, 15 developed regional recurrence exclusively, and 1 patient developed a combination of local and regional recurrence. Distant metastasis occurred in 7 patients, of which 6 occurred in the lung; 7 patients developed both distant metastasis and locoregional recurrence concomitantly. Mean time to locoregional recurrence was 1.52 years (range 0.35-11.48 years); mean time to distant metastasis was 0.70 years and to combined recurrence 2.26 years. Among the patients which developed local recurrence only, 11 underwent surgical salvage, 7 received systemic treatment (chemotherapy, immunotherapy, or chemo-immunotherapy) and 10 received palliative treatment. Among the patients that developed regional recurrence 12 underwent survival salvage, 3 received palliative treatment. Patients with isolated distant metastatic disease underwent surgical salvage (n=2; lung resection), systemic treatment (n=4) or palliative treatment (n=1). Among patients with multisite recurrence, 2 underwent surgical salvage followed by systemic treatment and 5 underwent palliative treatment.
Table 1.
Patient and tumor characteristics.
| Age | Mean (years) | 59.5 |
|---|---|---|
| Gender | Male | 56 |
| Female | 0 | |
| Tobacco | Yes | 50 |
| No | 6 | |
| Pack years | Mean | 41.4 |
| Median | 40 | |
| T-classification | 1 | 10 |
| 2 | 16 | |
| 3 | 17 | |
| 4 | 13 | |
| N-classification | 0 | 11 |
| 1 | 4 | |
| 2a | 4 | |
| 2b | 18 | |
| 2c | 16 | |
| 3 | 3 |
p16 and p53 Concordance Between Primary and Recurrent/Metastatic Disease
Quantitative p16 immunohistochemistry was used to categorize 41 tumor pairs as p16 positive (+) or p16 negative (-) based on the current clinical standard of >70% strong nuclear and cytoplasmic staining [25]: 14/41 (34%) primary tumors and 10/41 (24%) recurrent tumors were p16+. Eight tumors maintained p16+ status at time of recurrence. Twenty-three tumors maintained p16- negative status at time of recurrence (Figure 1A). Six tumors converted from p16+ to p16- and 2 tumors converted from p16- (detectable but <70%) to p16+ (>90%) (Figure 1B). Among the tumors which converted from p16+ to p16-, one was a primary site recurrence 8 years post initial treatment and 5 were nodal recurrences which occurred <24months following treatment completion. One primary tumor converted from p16- to p16+ 7 years later and one p16- primary generated a p16+ lung metastasis <1 year following treatment. Overall, we detected a very high rate of p16 concordance between primary and recurrent disease. To validate the quantitative p16 IHC data, we analyzed the tumors for high-risk HPV mRNA. Of the 41 primary tumors with both p16 and HPV data, concordance was 91%; of the 43 recurrent tumors with both p16 and HPV data, concordance was 95%. All 6 discordant tumors among the 84 analyzed had p16 staining >70% but negative HPV staining. Five of 6 tumors which converted from p16+ to p16- were verified by the HPV mRNA staining. The one outlier retained p16 positivity in the recurrence, but did not reach the 70% threshold and HPV was negative in both the primary and recurrent tumors.
Although TP53 sequencing is the gold standard for determining mutational status for this critical tumor suppressor, p53 IHC has demonstrated high concordance with mutational analysis across multiple tumor sites and patient cohorts [27–29]. Forty tumor pairs were analyzed in parallel for p53 and p16 expression levels. We identified 24 primary tumors as likely TP53 mutant based on staining patterns along with 22 recurrent tumors; there were 15 concordant mutant-mutant pairs and 9 concordant wildtype-wildtype pairs (Figure 1A, 1D). Among tumors with >80% p53 positive cells, none demonstrated positive p16 status; the concordance decreased for tumors with <5% p53 positive cells, with 8 tumors demonstrating both <5% p53 positive cells and p16 positivity.
Multinucleation Concordance Between Primary and Recurrent/Metastatic Disease
Multinucleation scores (MuNI) were analyzed for 47 tumor pairs. Mean MuNI scores were 0.00032 for primary tumors (range 0-0.00083) with mean MuNI score for recurrent tumors of 0.00037 (0-0.00062). We previously defined two MuNI thresholds as a function of survival, one of 0.00038 based on a large, multi-institutional OPSCC patient cohort [17] and a second threshold recently defined by our group at 0.00046 based on tumor biology (threshold defines >90% of HPV- associated tumors) [30]. Using the 0.00046 cutoff, 9 primary tumors were considered high-risk and 15 recurrences were considered high-risk. Across the time period, 19 tumors remained low-risk (40%), 14 converted from low-risk to high-risk (30%), 7 remained high-risk (15%) and 7 converted from high-risk to low-risk (15%). When limiting the analysis to primary site recurrences only (25), 10 tumors remained low-risk (40%), 7 converted to high-risk (28%), 4 remained high-risk (16%) and 4 (16%) converted to low-risk. Differences in MuNI between primary and recurrent tumors did not reach significance across all tumors (paired t-test 0.07), when limited to just primary site recurrences (0.084) or when limited to primary + regional recurrence (0.06). This was confirmed using chi-square analysis dichotomizing tumors as low-risk vs high-risk using either the 0.00046 or the 0.00038 thresholds.
Immune Infiltration Concordance Between Primary and Recurrent/Metastatic Disease
We utilized 2 overlapping approaches to measure immune infiltration of paired tumors. First, we quantitatively assessed CD3+ and CD8+ TIL levels in the tumors as previously described [8]. Mean CD3+ infiltration was 10.7% and 10.9% of primary and recurrent tumors (expressed as a % of total intra-tumoral nuclei) (paired t-test p = 0.96); mean CD8+ infiltration was 5.9% and 9.9%, respectively (paired t-test p = 0.025) (Figure 2). Mean CPS was 2.4 for primary tumors and 4.0 for recurrent tumors (paired t-test p = 0.04). Eight primary tumors and 21 recurrent tumors were positive for PD-L1. Only 1 primary tumor converted from positive to negative upon recurrence. All other tumors retained positivity (Figure 2).
Second, we calculated OP-TIL scores for primary and recurrent tumors as previously outlined [18] and dichotomized tumors into high-risk and low-risk. Chi-square analysis demonstrated no difference in OP-TIL scores between primary and recurrent tumors (chi-square statistic is 2.26; p-value = 0.13). When combining MuNI and OP-TIL scores for primary and recurrent tumors, chi-square analysis again demonstrated no significant difference between primary and recurrent tumors (chi-square statistic is 0.09; p-value = 0.77). OP-TIL status changed (high- to low- risk or vice versa) in only 18/46 pairs.
Discussion
Current approaches to the treatment of head and neck cancer are predicated on the basic concept that treatment delivery, whether consisting of chemotherapy, radiation or chemo-radiation, alters the intrinsic biological background of the tumor along with the tumor immune microenvironment. For radiation-based treatment, repeated application of radiation is limited to use for treatment of patients whose tumors demonstrate recurrence after a prolonged disease-free interval [31, 32] in large part due to the high fraction of patients who either develop ≥ Grade 3 toxicity and or have limited survival benefit. In both oropharyngeal and laryngeal squamous cell carcinoma patients, surgical salvage efficacy is strongly correlated with disease-free interval following initial treatment completion [33, 34]. Disease-free interval is also considered in the context of re-application of cytotoxic chemotherapy combined with immune checkpoint inhibitors. In CheckMate 141, patients with recurrent disease were referred to as “platinum refractory”, and the study utilized tumor progression or recurrence within 6 months of last platinum administration as an inclusion criteria for nivolumab administration [35]. Conversely, KEYNOTE-048 which tested pembrolizumab alone or in combination with cytotoxic chemotherapy compared to cytotoxic chemotherapy alone excluded patients which had progressive disease within 6 months of curatively intended systemic treatment for locoregionally advanced disease [36].
Driving these clinical decisions is the assumption that a tumor, previously exposed to chemo-radiation, that persists after treatment cessation will demonstrate molecular features of chemo-radioresistance, which would limit the expected utility of additional treatment. Our data shed some light on whether delivery of curative intent chemo-radiation impacts tumor biology through two phenotypic features we previously linked to oncologic outcomes, namely anti-tumor immunity and tumor cell multinucleation. Overall, tumors remained stable following treatment, without an upward drift in multinucleation nor a downward drift in TIL infiltration when assessed using quantitative immunohistochemistry for CD3+ and cytotoxic CD8+ subsets. Recurrent tumors, in fact, demonstrated slightly higher levels of CD8+ TILs and higher CPS. Although CD8+ TIL levels and CPS were higher in recurrent tumors, the OP-TIL algorithm, which combines TIL quantity with overall distribution and spatial organization within the tumor compartment, detected no significant differences between primary and recurrent tumors.
In contrast to our findings, So et al. [37] used paired tumor analysis combined with IHC and mRNA profiling to show that CD8+ TIL and PD-L1 expression levels decreased in a majority of recurrent head and neck squamous cell carcinoma (HNSCC) tumors compared to the primary tumors (although both parameters varied widely). A more focused approach was used by Pflumio et al. [38] to evaluate recurrence within a previously irradiated HNSCC field and showed lower CD3+ TIL levels and PD-L1 expressing cells in irradiated tumors but consistent CD8+TIL levels. These discordant results explain in part the lack of consistent PD-L1-based CPS scoring trends between primary and recurrent tumors summarized by Girolami et al. [39] in a systematic analysis of the literature. Discordant datasets may in part be due to inclusion of different HNSCC sites (e.g., oral cavity, oropharynx, larynx) and variable HPV association. Park et al. [40] recently used paired local recurrences for chemo-radiation treated HNSCC to show discordance of PD-L1 varied as a function of site with oral cavity demonstrating higher discordance and variable discordance depending on the CPS threshold chosen for analysis. Even when the selection for low levels of PD-L1 is maximal, as in the context of clinical trials utilizing anti-PD1/PD-L1 antibody-based strategies, concordance of anti-tumor immunity, whether measured via PD-L1 IHC or gene expression (e.g., interferon gamma, tertiary lymphoid structure signatures) is variable between primary and recurrent tumors [41].
Intrinsic biological characteristics measured via p16 and p53 IHC appeared concordant between primary and recurrent tumors analyzed in the current study in contrast to data from several other studies (not limited to OPSCC) which showed reduction in mucin (MUC) 1 expression in recurrent tumors [42] along with higher rates of p14(ARF) alterations (promoter methylation, point mutations) [43], and a discordant TP53 mutational pattern including shifts from wild-type to mutant status and loss of TP53 mutation at time of disease recurrence [44]. A very detailed analysis by Hedberg et al. [45] found an overall higher rate of primary and synchronous metastasis concordance using whole exome sequencing compared to primary tumors and their recurrent counterparts including enrichment of discoidin domain receptor tyrosine kinase 2 (DDR2) events.
A fundamental biological question for the oropharyngeal subset of head and neck squamous cell carcinomas is that of HPV association. The current clinical standard for this disease site, which was utilized here, is p16 immunohistochemistry. However, since this is a surrogate marker, we sought to confirm HPV association using direct testing for HPV. As summarized above, direct HPV testing and p16 IHC were concordant in over 90% of both primary and recurrent tumors although the concordance did not reach 100%. The discordance occurred in a single direction, namely high p16 levels but undetectable HPV levels which might reflect methodological limitations. Importantly, changes in p16 within individual tumor pairs, were confirmed by direct HPV testing which speaks to the validity of a biological shift between the primary tumor and the recurrent disease following treatment.
Limitations of the current study include limited sample size and a limited scope of analysis as opposed to a more in depth transcriptomic and/or genomic analysis. However, in contrast to previous studies, ours is the first to limit the analysis to one HNSCC site, namely oropharynx and at the same time focus on patients who underwent curative intent radiation-based treatment. We focused our analysis on multinucleation and anti-tumor immunity because they represent previously validated phenotypic markers of treatment response and or effectiveness in the setting of primary OPSCC treated with either surgery or chemo-radiation [8, 16–18, 30]. As a result, we expected that significant differences in these markers would provide more proximate phenotypic information about primary and recurrent disease. Future studies will expand both the number of paired samples and the depth of the analysis using spatial transcriptomic approaches that will allow us to not only better characterize potential changes in the tumor immune microenvironment but further unravel the relationship between multinucleation and infiltrating immunocytes. Because of the limited sample size, our analysis did not separate the tumor pairs into HPV-associated and HPV-independent. For a detailed analysis of the non-HPV associated parameters measured here, namely multinucleation, immune infiltration and p53 expression levels, we would propose a larger cohort dichotomized by combined fully concordant p16+ / HPV+ vs p16- / HPV- status that could shed additional information on the whether tumor biology remains relatively stable during/following treatment or whether treatment generates dramatic shifts in tumor biology. Ongoing collaborations with several other institutions are designed to build this larger and more robust cohort for future analysis.
One limitation of the current study, shared by all other similar work stems from the nature of solid tumors themselves. Whereas in hematopoietic malignancies, interrogation of blasts during and immediately post-treatment makes it possible to rapidly assess development of resistant molecular backgrounds, in solid tumors, we are limited to sampling tumors prior to treatment and once the recurrent disease becomes clinically evident. Even when this process is temporally compressed, the number of population doublings between the end of treatment and development of measurable (~1cm) disease or persistence of viable tumor at first post-treatment scan (2-3 months post-treatment completion) is massive. This extended duration raises the possibility that the impact of treatment on tumor biology may be diluted during subsequent tumor expansion. To overcome this intrinsic limitation, our ongoing studies couple conventional analysis outlined here with serial interrogation of cell free DNA and circulating exosome to continuously interrogate tumor biology during and following treatment.
Conclusions
Data from the current study do not support a significant deleterious shift in either intrinsic tumor biology or anti-tumor immunity (assessed within the tumor volume) that could negatively impact the effectiveness of salvage therapeutic strategies.
Author contributions
PC, GC, CK, LAN, TJ, TL collected and analyzed the data. JSL, AM, MJF, VCS supervised data analysis and provided quality assurance for all data collection and analysis. PC, GC, CK, JSL, AM, MJ, VCS drafted the manuscript. All authors reviewed and participated in the revision process for the manuscript. PC and VCS prepared the figures.
Funding
This work was supported by a Career Development Award to VCS from the Veterans Administration Clinical Science Research and Development division (1IK2CX001953) and through the Dan L Duncan Comprehensive Cancer Center (P30-CA125123) and Human Tissue Acquisition & Pathology Core Baylor College of Medicine. Dr Madabhushi’s work is supported from grants supported by the National Cancer Institute under award numbers 1U24CA199374-01, R01CA249992-01A1, R01CA202752-01A1, R01CA208236-01A1, R01CA216579-01A1, R01CA220581-01A1, R01CA257612-01A1, 1U01CA239055-01, 1U01CA248226-01, 1U54CA254566-01, National Heart, Lung and Blood Institute 1R01HL15127701A1, R01HL15807101A1, National Institute of Biomedical Imaging and Bioengineering 1R43EB028736-01, National Center for Research Resources under award number 1 C06 RR12463-01; VA Merit Review Award IBX004121A from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Service, the Office of the Assistant Secretary of Defense for Health Affairs, through the Breast Cancer Research Program (W81XWH-19-1-0668), the Prostate Cancer Research Program (W81XWH-15-1-0558, W81XWH-20-1-0851), the Lung Cancer Research Program (W81XWH-18-1-0440, W81XWH-20-1-0595), the Peer Reviewed Cancer Research Program (W81XWH-18-1-0404, W81XWH-21-1-0345), the Kidney Precision Medicine Project (KPMP) Glue Grant, the Ohio Third Frontier Technology Validation Fund, the Mayo Clinic Breast Cancer SPORE grant P50 CA116201 from the National Institutes of Health, the Clinical and Translational Science Collaborative of Cleveland (UL1TR0002548) from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, The Wallace H. Coulter Foundation Program in the Department of Biomedical Engineering at Case Western Reserve University, sponsored research agreements from Bristol Myers-Squibb, Boehringer-Ingelheim, and AstraZeneca.
Declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
There are no conflicts of interest directly related to this work. Dr. Sandulache is a consultant for Femtovox Inc. Dr. Madabhushi is an equity holder in Picture Health, Elucid Bioimaging, and Inspirata Inc. Currently he serves on the advisory board of Picture Health, Aiforia Inc, and SimBioSys. He also currently consults for Roche, Biohme, and Castle Biosciences. He also has sponsored research agreements with AstraZeneca, Boehringer-Ingelheim, Eli-Lilly and Bristol Myers-Squibb. His technology has been licensed to Picture Health and Elucid Bioimaging. He is also involved in 3 different R01 grants with Inspirata Inc.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gravitt PE, Winer RL. Natural history of HPV infection across the lifespan: role of viral latency. Viruses. 2017 doi: 10.3390/v9100267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta SM, Mania-Pramanik J. Molecular mechanisms in progression of HPV-associated cervical carcinogenesis. J Biomed Sci. 2019;26(1):28. doi: 10.1186/s12929-019-0520-2. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Kreimer AR, Villa A, Nyitray AG, et al. The epidemiology of oral HPV infection among a multinational sample of healthy men. Cancer Epidemiol Biomark Prev. 2011;20(1):172–82. doi: 10.1158/1055-9965.EPI-10-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castellsague X. Natural history and epidemiology of HPV infection and cervical cancer. Gynecol Oncol. 2008;110(3 Suppl 2):S4–S7. doi: 10.1016/j.ygyno.2008.07.045. [DOI] [PubMed] [Google Scholar]
- 5.Lechner M, Liu J, Masterson L, Fenton TR. HPV-associated oropharyngeal cancer: epidemiology, molecular biology and clinical management. Nat Rev Clin Oncol. 2022;19(5):306–327. doi: 10.1038/s41571-022-00603-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahlstrom KR, Calzada G, Hanby JD, et al. An evolution in demographics, treatment, and outcomes of oropharyngeal cancer at a major cancer center: a staging system in need of repair. Cancer. 2013;119(1):81–9. doi: 10.1002/cncr.27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandulache VC, Hamblin J, Lai S, et al. Oropharyngeal squamous cell carcinoma in the veteran population: association with traditional carcinogen exposure and poor clinical outcomes. Head Neck. 2015;37(9):1246–53. doi: 10.1002/hed.23740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilde DC, Castro PD, Bera K, et al. Oropharyngeal cancer outcomes correlate with p16 status, multinucleation and immune infiltration. Mod Pathol. 2022 doi: 10.1038/s41379-022-01024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zevallos JP, Kramer JR, Sandulache VC, et al. National trends in oropharyngeal cancer incidence and survival within the Veterans Affairs Health Care System. Head Neck. 2021;43(1):108–115. doi: 10.1002/hed.26465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elhalawani H, Mohamed ASR, Elgohari B, et al. Tobacco exposure as a major modifier of oncologic outcomes in human papillomavirus (HPV) associated oropharyngeal squamous cell carcinoma. BMC Cancer. 2020;20(1):912. doi: 10.1186/s12885-020-07427-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vawda N, Banerjee RN, Debenham BJ. Impact of smoking on outcomes of HPV-related oropharyngeal cancer treated with primary radiation or surgery. Int J Radiat Oncol Biol Phys. 2019;103(5):1125–1131. doi: 10.1016/j.ijrobp.2018.11.046. [DOI] [PubMed] [Google Scholar]
- 12.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fakhry C, Zhang Q, Gillison ML, et al. Validation of NRG oncology/RTOG-0129 risk groups for HPV-positive and HPV-negative oropharyngeal squamous cell cancer: Implications for risk-based therapeutic intensity trials. Cancer. 2019;125(12):2027–2038. doi: 10.1002/cncr.32025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fakhry C, Zhang Q, Nguyen-Tan PF, et al. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J Clin Oncol. 2014;32(30):3365–73. doi: 10.1200/JCO.2014.55.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillison ML, Zhang Q, Jordan R, et al. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol. 2012;30(17):2102–11. doi: 10.1200/JCO.2011.38.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koyuncu CF, Nag R, Lu C, et al. Image analysis reveals differences in tumor multinucleations in Black and White patients with human papillomavirus-associated oropharyngeal squamous cell carcinoma. Cancer. 2022;128(21):3831–3842. doi: 10.1002/cncr.34446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koyuncu CF, Lu C, Bera K, et al. Computerized tumor multinucleation index (MuNI) is prognostic in p16+ oropharyngeal carcinoma: a multi-site validation study. J Clin Invest. 2021 doi: 10.1172/JCI145488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corredor G, Toro P, Koyuncu C, et al. An imaging biomarker of tumor-infiltrating lymphocytes to risk-stratify patients with HPV-associated oropharyngeal cancer. J Natl Cancer Inst. 2021 doi: 10.1093/jnci/djab215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kemnade JO, Elhalawani H, Castro P, et al. CD8 infiltration is associated with disease control and tobacco exposure in intermediate-risk oropharyngeal cancer. Sci Rep. 2020;10(1):243. doi: 10.1038/s41598-019-57111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandulache VC, Michikawa C, Kataria P, et al. High-risk TP53 mutations are associated with extranodal extension in oral cavity squamous cell carcinoma. Clin Cancer Res. 2018;24(7):1727–1733. doi: 10.1158/1078-0432.CCR-17-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wahle BM, Zolkind P, Ramirez RJ, et al. Integrative genomic analysis reveals low T-cell infiltration as the primary feature of tobacco use in HPV-positive oropharyngeal cancer. iScience. 2022;25(5):104216. doi: 10.1016/j.isci.2022.104216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zanoni DK, Patel SG, Shah JP (2019) Changes in the 8th Edition of the American Joint Committee on Cancer (AJCC) Staging of Head and Neck Cancer: Rationale and Implications. Curr Oncol Rep 21(6):52. 10.1007/s11912-019-0799-x [DOI] [PMC free article] [PubMed]
- 23.Yemelyanova A, Vang R, Kshirsagar M, et al. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Mod Pathol. 2011;24(9):1248–53. doi: 10.1038/modpathol.2011.85. [DOI] [PubMed] [Google Scholar]
- 24.Kobel M, Kang EY. The many uses of p53 immunohistochemistry in gynecological pathology: proceedings of the ISGyP companion society session at the 2020 USCAP Annual9 Meeting. Int J Gynecol Pathol. 2021;40(1):32–40. doi: 10.1097/PGP.0000000000000725. [DOI] [PubMed] [Google Scholar]
- 25.Lewis JS, Jr, Beadle B, Bishop JA, et al. Human Papillomavirus testing in head and neck carcinomas: guideline from the College of American Pathologists. Arch Pathol Lab Med. 2018;142(5):559–597. doi: 10.5858/arpa.2017-0286-CP. [DOI] [PubMed] [Google Scholar]
- 26.Janowczyk A, Zuo R, Gilmore H, Feldman M, Madabhushi A. HistoQC: an open-source quality control tool for digital pathology slides. JCO Clin Cancer Inform. 2019;3:1–7. doi: 10.1200/CCI.18.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sung YN, Kim D, Kim J. p53 immunostaining pattern is a useful surrogate marker for TP53 gene mutations. Diagn Pathol. 2022;17(1):92. doi: 10.1186/s13000-022-01273-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruzinova MB, Lee YS, Duncavage EJ, Welch JS. TP53 immunohistochemistry correlates with TP53 mutation status and clearance in decitabine-treated patients with myeloid malignancies. Haematologica. 2019;104(8):e345–e348. doi: 10.3324/haematol.2018.205302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park E, Han H, Choi SE, et al. p53 immunohistochemistry and mutation types mismatching in high-grade serous ovarian cancer. Diagnostics (Basel) 2022 doi: 10.3390/diagnostics12030579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koyuncu CF, Frederick MJ, Thompson LDR, et al. Machine learning driven index of tumor multinucleation correlates with survival and suppressed anti-tumor immunity in head and neck squamous cell carcinoma patients. Oral Oncol. 2023;143:106459. doi: 10.1016/j.oraloncology.2023.106459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ward MC, Riaz N, Caudell JJ, et al. Refining patient selection for reirradiation of head and neck squamous carcinoma in the IMRT era: a multi-institution cohort study by the MIRI collaborative. Int J Radiat Oncol Biol Phys. 2018;100(3):586–594. doi: 10.1016/j.ijrobp.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ward MC, Lee NY, Caudell JJ, et al. A competing risk nomogram to predict severe late toxicity after modern re-irradiation for squamous carcinoma of the head and neck. Oral Oncol. 2019;90:80–86. doi: 10.1016/j.oraloncology.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 33.Sandulache VC, Vandelaar LJ, Skinner HD, et al. Salvage total laryngectomy after external-beam radiotherapy: a 20-year experience. Head Neck. 2016;38(Suppl 1):E1962–8. doi: 10.1002/hed.24355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zafereo ME, Hanasono MM, Rosenthal DI, et al. The role of salvage surgery in patients with recurrent squamous cell carcinoma of the oropharynx. Cancer. 2009;115(24):5723–33. doi: 10.1002/cncr.24595. [DOI] [PubMed] [Google Scholar]
- 35.Ferris R, Gillison ML. Nivolumab for squamous-cell cancer of head and neck. N Engl J Med. 2017;376(6):596. doi: 10.1056/NEJMc1615565. [DOI] [PubMed] [Google Scholar]
- 36.Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 37.So YK, Byeon SJ, Ku BM, et al. An increase of CD8(+) T cell infiltration following recurrence is a good prognosticator in HNSCC. Sci Rep. 2020;10(1):20059. doi: 10.1038/s41598-020-77036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pflumio C, Thomas J, Salleron J, et al. Expression of immune response biomarkers (PD-L1, p16, CD3+ and CD8+ TILs) in recurrent head and neck squamous cell carcinoma within previously irradiated areas. Oncol Rep. 2021;45(3):1273–1283. doi: 10.3892/or.2021.7928. [DOI] [PubMed] [Google Scholar]
- 39.Girolami I, Marletta S, Fiorentino V, et al. Effect of radio-chemotherapy on PD-L1 immunohistochemical expression in head and neck squamous cell carcinoma. J Pers Med. 2023 doi: 10.3390/jpm13020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park BJ, Mattox AK, Clayburgh D, et al. Chemoradiation therapy alters the PD-L1 score in locoregional recurrent squamous cell carcinomas of the head and neck. Oral Oncol. 2022;135:106183. doi: 10.1016/j.oraloncology.2022.106183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karabajakian A, Bouaoud J, Michon L, et al. Longitudinal assessment of PD-L1 expression and gene expression profiles in patients with head and neck cancer reveals temporal heterogeneity. Oral Oncol. 2021;119:105368. doi: 10.1016/j.oraloncology.2021.105368. [DOI] [PubMed] [Google Scholar]
- 42.Croce MV, Rabassa ME, Pereyra A, Segal-Eiras A. Differential expression of MUC1 and carbohydrate antigens in primary and secondary head and neck squamous cell carcinoma. Head Neck. 2008;30(5):647–57. doi: 10.1002/hed.20756. [DOI] [PubMed] [Google Scholar]
- 43.Weber A, Bellmann U, Bootz F, Wittekind C, Tannapfel A. INK4a-ARF alterations and p53 mutations in primary and consecutive squamous cell carcinoma of the head and neck. Virchows Arch. 2002;441(2):133–42. doi: 10.1007/s00428-002-0637-6. [DOI] [PubMed] [Google Scholar]
- 44.Weber A, Bellmann U, Bootz F, Wittekind C, Tannapfel A. Expression of p53 and its homologues in primary and recurrent squamous cell carcinomas of the head and neck. Int J Cancer. 2002;99(1):22–8. doi: 10.1002/ijc.10296. [DOI] [PubMed] [Google Scholar]
- 45.Hedberg ML, Goh G, Chiosea SI, et al. Genetic landscape of metastatic and recurrent head and neck squamous cell carcinoma. J Clin Invest. 2016;126(4):1606. doi: 10.1172/JCI86862. [DOI] [PMC free article] [PubMed] [Google Scholar]