Abstract
Background
Studies with methodological advancements are warranted to confirm the relation of red meat consumption to the incidence of type 2 diabetes (T2D).
Objective
We aimed to assess the relationships of intakes of total, processed, and unprocessed red meat to risk of T2D and to estimate the effects of substituting different protein sources for red meats on T2D risk.
Methods
Our study included 216,695 participants (81% females) from the Nurses’ Health Study (NHS), NHS II, and Health Professionals Follow-up Study (HPFS). Red meat intakes were assessed with semiquantitative food frequency questionnaires (FFQs) every 2 to 4 y since the study baselines. We used multivariable-adjusted proportional hazards models to estimate the associations between red meats and T2D.
Results
Over 5,483,981 person-years of follow-up, we documented 22,761 T2D cases. Intakes of total, processed, and unprocessed red meat were positively and approximately linearly associated with higher risks of T2D. Comparing the highest to the lowest quintiles, hazard ratios (HR) were 1.62 (95% confidence interval [CI]: 1.53, 1.71) for total red meat, 1.51 (95% CI: 1.44, 1.58) for processed red meat, and 1.40 (95% CI: 1.33, 1.47) for unprocessed red meat. The percentage lower risk of T2D associated with substituting 1 serving/d of nuts and legumes for total red meat was 30% (HR = 0.70, 95% CI: 0.66, 0.74), for processed red meat was 41% (HR = 0.59, 95% CI: 0.55, 0.64), and for unprocessed red meat was 29% (HR = 0.71, 95% CI: 0.67, 0.75); Substituting 1 serving/d of dairy for total, processed, or unprocessed red meat was also associated with significantly lower risk of T2D. The observed associations became stronger after we calibrated dietary intakes to intakes assessed by weighed diet records.
Conclusions
Our study supports current dietary recommendations for limiting consumption of red meat intake and emphasizes the importance of different alternative sources of protein for T2D prevention.
Keywords: red meat, processed red meat, unprocessed red meat, type 2 diabetes, calibration, sources of protein, substitution
Introduction
Type 2 diabetes (T2D) is a major public health concern globally, and both the incidence and prevalence are increasing rapidly [1, 2]. In observational studies, red meat intake has been associated with risk of type 2 diabetes (T2D) [3, 4], and replacement of red meat with other protein sources has been associated with lower risk in statistical substitution analyses [[5], [6], [7], [8], [9]]. However, in short-term randomized controlled trials (RCTs), definitive effects of red meat intake on biomarkers of glycemic control or inflammation have not been seen [10, 11]. Challenges to the quality of observational studies and reinterpretation of existing evidence have been used to counter recommendations to limit the consumption of red meat [12, 13]. That long-term RCTs of red meat intake and incident T2D might never be conducted has been acknowledged, in part due to lack of clinical equipoise and feasibility because T2D may take decades to develop [9]. Therefore, long-term observational studies with methodological advancements are warranted to evaluate the relation of red meat consumption to the incidence of T2D.
In previous analyses in the Nurses’ Health Study (NHS), Nurses’ Health Study II (NHS II), and Health Professionals Follow-up Study (HPFS), a strong positive association between red meat, particularly processed red meat, and incident T2D was observed [4]. In the current analysis, we aimed to investigate further this association in the same 3 cohorts, with over 9000 additional T2D cases documented with extended follow-up. This large number of cases and repeated dietary assessments over 30 y provides a precise estimation of the relationship between red meat and T2D, the degree of attenuation due to using a single assessment of diet as in most epidemiologic studies, and the effects of substituting other protein sources for red meats. This also allows an examination of latency periods and potential reverse causation. Moreover, with the availability of 2 large calibration studies [14, 15], we calibrated self-reported red meat intake with weighed diet records for the first time. We hypothesized that higher red meat intake is associated with a higher risk of T2D and that substitution of fish, dairy, nuts and legumes, poultry, or eggs for red meat is associated with a lower risk of T2D.
Methods
Study population
The present study included participants from 3 prospective cohort studies: the NHS, NHS II, and HPFS. The NHS recruited 121,700 female registered nurses aged 30 to 55 y in 1976 from 11 US states. The NHS II was initiated in 1989 and enrolled 116,429 female registered nurses aged 25 to 42 y from 14 US states. The HPFS is a national study of 51,529 male veterinarians, dentists, pharmacists, optometrists, osteopath physicians, and podiatrists aged 40 to 75 y at recruitment in 1986. Participants of the 3 cohorts have been followed biennially through mailed questionnaires about diseases and health-related topics. In our analyses, we used the year when the dietary data were first collected as the baseline of each cohort (1980 for NHS, 1991 for NHS II, and 1986 for HPFS). Detailed study designs of the NHS, NHS II, and HPFS were described elsewhere [16, 17]. The study protocols were approved by the institutional review boards of Brigham and Women’s Hospital and the Harvard School of Public Health. Participants’ consent was implied by the return of the questionnaires.
We excluded participants who reported a baseline history of diabetes, myocardial infarction, angina, stroke, coronary artery bypass grafting, or cancer. Female participants who had an energy intake of <500 kcal/d or >3500 kcal/d, male participants who had an energy intake of <800 kcal/d or >4200 kcal/d, and female or male participants who had missing age or baseline red meat intake were also excluded. The final analytical population included 84,315 females from the NHS, 90,217 females from the NHS II, and 42,163 males from the HPFS (Supplemental Figure 1).
Exposure and covariates assessment
Dietary intakes were assessed every 2 to 4 y using semiquantitative food frequency questionnaires (FFQs); the 1980 FFQ used in the NHS included 61 items; in 1984 and thereafter, this was expanded to approximately 120 items. On the FFQs, participants were asked to report their average intake of each food or beverage over the past 12 mo. For each food or beverage, the portion size was specified, and frequency choices ranged from <1 time/mo to ≥6 times/d. The reproducibility and validity of the FFQs used in our study and red meats assessed by FFQs were reported previously and in Supplemental Table 1 [18, [19], [25]]. Total red meat intake was computed as the sum of serving intakes of processed and unprocessed red meats. Processed red meats included beef or pork hot dogs; bacon; processed meat sandwiches; and other processed meats such as sausage. Unprocessed red meats included lean or extra lean hamburger; regular hamburger; beef, pork, or lamb as a sandwich or mixed dish; pork as a main dish; and beef or lamb as a main dish. The composition of other food groups, such as fish, dairy, nuts and legumes, poultry, and eggs, are described in Supplemental Table 2. Total energy intake and alcohol intake were computed based on nutrient contents from the Harvard University Food Composition Database [20]. The Alternative Health Eating Index (AHEI-2010), as an assessment of the overall healthfulness of diet, and glycemic index were also calculated from foods and nutrient intakes collected with FFQs. For our main analyses, we calculated cumulative averages of dietary intakes from the baseline FFQ to the start of each 2 or 4-y follow-up interval to reduce random measurement error from within-person variations of diet and to capture dietary changes over time. For example, in the NHS, red meat intakes in 1980, 1984, 1986, and 1990 were averaged to predict risk of diabetes from 1990 to 1994.
Date of birth, race/ethnicity, height, family history of T2D, and history of hypertension were collected either at baseline or during the follow-up. Information on body weight, smoking status, physical activity, multivitamin use, menopausal status and hormone use (if NHS or NHS II), antihypertensive drug use, and cholesterol-lowering drug use were updated every 2 to 4 y through questionnaires. Individual-level and area-level socioeconomic status was characterized by a geo-coded composite score of participants’ education, income, house value, and marital status [21].
Diabetes incidence
In all cohorts, incident T2D diagnoses were reported by participants through biennial questionnaires. A supplementary questionnaire regarding symptoms, diagnostic tests, and hypoglycemic therapy was then mailed to the participants who reported this diagnosis. A T2D case was confirmed if at least one of the following criteria from the American Diabetes Association was reported on the supplementary questionnaire: 1) one or more classic symptoms (excessive thirst, polyuria, weight loss, hunger, pruritus, or coma) plus fasting plasma glucose concentration ≥126 mg/dl or random plasma glucose concentration ≥200 mg/dl; 2) at least 2 elevated plasma glucose levels on different occasions (fasting plasma glucose ≥140 mg/dl and/or random plasma glucose ≥200 mg/dl and/or plasma glucose ≥200 mg/dl at ≥2 h on oral glucose tolerance testing) in the absence of symptoms; 3) treatment with hypoglycemic medication (insulin or oral hypoglycemic agent). For cases reported before 1998, fasting plasma glucose concentration ≥140 mg/dL was used for diabetes diagnosis, according to the National Diabetes Data Group [22]. The high validity of the self-reported T2D confirmed by the supplementary questionnaires was documented by comparison with medical records [23, 24].
Dietary calibration studies
To correct associations for errors in dietary assessment, we used dietary intakes assessed by both FFQs and 7-d weighed diet records (7DDRs) provided by 1207 males and females from the Women’s Lifestyle Validation Study (WLVS) and Men’s Lifestyle Validation Study (MLVS). Participants of WLVS and MLVS were recruited as subsets of NHS, NHS II, and HPFS participants and members of a Boston-area health plan [14, 15, 25]. FFQs were administered at the baseline and end of the 1-y study period. Two 7DDRs, as the reference assessments that do not rely on memory and, thus, have errors mostly independent of those associated with the FFQs, were collected approximately 6 mo apart within the study period.
Statistical analysis
In our analysis, the end of follow-up was defined as 30 June 2016 for the NHS, 30 June 2017 for the NHS II, and 31 January 2016 for the HPFS. Person-years of follow-up were calculated from the return of the baseline questionnaire until the earliest time of T2D diagnosis, death, loss to follow-up, or the end of follow-up. We used Cox proportional hazards models to estimate hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) for the associations of total red meat, processed red meat, and unprocessed red meat with the incidence of T2D. The proportional hazards models were stratified jointly by age in months and calendar time in 2-y groups and were adjusted for race/ethnicity, smoking status, alcohol intake, physical activity [metabolic equivalents (METs)-hours/week], multivitamin use, menopausal status and hormone use (if in NHS or NHS II), family history of T2D, antihypertensive drug use, cholesterol-lowering drug use, baseline history of hypertension, dietary glycemic index, poultry, fish, egg, total dairy, nuts and legumes, fruits, vegetables, whole grain, and refined grain intakes, and socioeconomic status. We modeled total red meat, processed red meat, and unprocessed red meat intakes both categorically (as quintiles and as 8 even-increment intake categories) and continuously (as a serving/d increment). In the categorical analyses, we quantified linear trends across categories by modeling the median of each quintile or category of red meat intake continuously and used the Wald test to evaluate statistical significance. We examined the possibly nonlinear relation between red meats and risk of T2D with restricted cubic splines [26]. Tests for nonlinearity used the likelihood ratio test, comparing the model with only the linear term to the model with the linear and the cubic spline terms.
BMI was not adjusted in the primary analysis, considering its potential mediating role in the associations between red meat intakes and T2D. We estimated the percentage of the association between red meat intake and risk of diabetes that is statistically accounted for by BMI in a sensitivity analysis. However, to address potential residual confounding due to BMI, we further adjusted time-varying BMI and baseline BMI in secondary analyses. We also evaluated the associations between red meat intakes and T2D risk in subgroups defined by physical activity levels (< median METs-h/wk vs. median METs- h/wk), BMI (< 25 kg/m2, 25–30 kg/m2, and 30 kg/m2), hypertension at baseline, smoking status (never, past, and current), and race/ethnicity defined based on National Institute of Health-suggested categories (White adults, Black adults, Hispanic adults, Asian adults, and racial/ethnic minorities as Black, Hispanic, and Asian adults combined). The interactions between red meats and dichotomously defined stratification variables (e.g., baseline hypertension, racial/ethnic minorities) were tested using the Wald test with 1 degree of freedom. For BMI, smoking status, and race/ethnicity defined as White adults, Black adults, Hispanic adults, and Asian adults, their interactions with red meats were evaluated by performing likelihood ratio tests comparing models with and without their product terms with red meats. For a more thorough control of confounding, we mutually adjusted for processed and unprocessed red meats in a sensitivity analysis.
To assess latency periods and to evaluate potential bias due to reverse causation, we conducted a latency analysis assuming latency periods of 0 to 4 y, 4 to 8 y, 8 to 12 y, 12 to 16 y, 16 to 20 y, and 20 to 24 y for the associations between every 1 serving/d increment in red meats and T2D risks. For example, in the latency analysis assuming a latency period of 8 to 12 y, red meat intake in 1980 was used to predict risk of diabetes from 1988 to 1992. As a sensitivity analysis, we examined and compared the risks of T2D associated with red meats assessed only at baseline, simply updated over the follow-up, modeled as cumulative averages over the follow-up, as cumulative averages excluding the most recent assessment prior to T2D diagnosis, and as cumulative averages of the most recent 3 assessments prior to T2D diagnosis. This also allowed us to evaluate the impact of reverse causation on the associations of interest.
To estimate the effect of substituting 1 serving/d of other protein sources for red meats, we included both intakes as continuous variables in the same multivariable Cox model. The HRs [and 95% confidence intervals (CIs)] were estimated using the difference in the estimated coefficients (and their pooled variances) [27, 28].
For the investigation of T2D risks associated with red meat intakes, we calibrated red meats and other dietary covariates assessed by FFQs with those assessed by 7DDRs using regression calibration [29]. Regression calibration allows correction for measurement error in dietary intakes measured with FFQs by incorporating information from a validation study where a reference measure, such as 7DDRs, was collected. Using data from the WLVS and MLVS, we regressed red meats assessed by 7DDRs on intake assessed by FFQs using a two-part regression to account for intakes reported as zeros [30]. The first part of the two-part regression employed logistic regression to handle zero and nonzero data, whereas the second part used linear regression to model nonzero intakes. For the dietary covariates, we fitted intakes measured with 7DDRs and FFQs with linear regressions as the calibration model. Covariates adjusted in the main analyses were also included in the calibration models. To preserve model efficiency, dietary covariates were summarized as modified AHEIs that excluded components representing the exposures of interest. For each participant at each dietary assessment in the main study, the calibrated red meats were calculated by multiplying the predicted probabilities of having nonzero intakes from the first part model by the expected values of intakes from the second part model. We used Cox proportional hazards models to estimate the associations between calibrated red meats and T2D. To integrate uncertainty in the derivation of the calibration equations and estimation of the exposure-outcome associations, we estimated the 95% CIs through bootstrapping with 100 iterations.
All analyses except the stratification by race/ethnicity were conducted separately in each cohort. An inverse-variance-weighted meta-analysis was used to combine the results across cohorts [31]. The associations between red meats and T2D in race/ethnicity subgroups were estimated from a pooled population of 3 cohorts to preserve statistical power in subgroups. We performed the data analyses using SAS software version 9.4 (SAS Institute, Cary, North Carolina) and R version 4.2.0. Statistical tests were performed as 2-tailed at an α-level of 0.05.
Results
Over 5,483,981 person-years of follow-up, we documented 11,369 T2D cases in the NHS, 7,624 cases in the NHS II, and 3,768 cases in the HPFS. At baseline, the mean (SD) age of the participants was 46.1 (7.2) in the NHS, 36.1 (4.7) in the NHS II, and 53.0 (9.5) in the HPFS. In both females and males, those with higher total red meat intake had higher BMI and total energy intake, were less physically active, were more likely to be current smokers, and were less likely to use multivitamins (Table 1).
TABLE 1.
Baseline characteristics of the study populations
| Quintiles of Total Red Meat Intake (servings/d) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| NHS (1980, n = 84,315) |
NHS II (1991, n = 90,217) |
HPFS (1986, n = 42,163) |
|||||||
| Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | |
| n | 16,621 | 16,473 | 17,120 | 17,778 | 18,187 | 18,418 | 8,530 | 8,103 | 8,598 |
| Age (y) | 47.4 (7.2) | 45.7 (7.2) | 45.7 (7.1) | 36.2 (4.7) | 36.1 (4.7) | 35.9 (4.6) | 54.3 (9.6) | 52.9 (9.6) | 52.2 (9.2) |
| Total Calories (kcal/d) | 1202 (397) | 1521 (388) | 2032 (474) | 1504 (492) | 1736 (472) | 2206 (525) | 1684 (543) | 1902 (520) | 2497 (609) |
| Total Red Meat (servings/d) | 0.46 (0.18) | 1.27 (0.11) | 2.62 (0.71) | 0.21 (0.14) | 0.82 (0.08) | 1.95 (0.54) | 0.24 (0.15) | 1.01 (0.12) | 2.42 (0.76) |
| Processed Red Meat (servings /d) | 0.11 (0.09) | 0.31 (0.23) | 0.75 (0.56) | 0.04 (0.06) | 0.19 (0.12) | 0.50 (0.40) | 0.05 (0.07) | 0.30 (0.21) | 0.85 (0.63) |
| Unprocessed Red Meat (servings /d) | 0.35 (0.15) | 0.95 (0.25) | 1.87 (0.67) | 0.17 (0.12) | 0.63 (0.13) | 1.45 (0.51) | 0.18 (0.13) | 0.71 (0.22) | 1.57 (0.63) |
| Poultry (servings /d) | 0.46 (0.44) | 0.42 (0.35) | 0.42 (0.41) | 0.68 (0.55) | 0.68 (0.42) | 0.69 (0.45) | 0.64 (0.54) | 0.55 (0.42) | 0.54 (0.42) |
| Fish (servings /d) | 0.22 (0.27) | 0.16 (0.18) | 0.14 (0.16) | 0.25 (0.29) | 0.23 (0.20) | 0.22 (0.21) | 0.44 (0.39) | 0.31 (0.27) | 0.27 (0.25) |
| Egg (servings /d) | 0.37 (0.38) | 0.41 (0.37) | 0.50 (0.44) | 0.13 (0.19) | 0.17 (0.18) | 0.25 (0.25) | 0.20 (0.34) | 0.31 (0.35) | 0.52 (0.55) |
| Total dairy (servings /d) | 1.84 (1.37) | 1.84 (1.29) | 1.87 (1.33) | 2.13 (1.45) | 2.27 (1.44) | 2.45 (1.52) | 1.70 (1.38) | 1.91 (1.37) | 2.26 (1.60) |
| Nuts and legumes (servings /d) | 0.46 (0.60) | 0.45 (0.46) | 0.51 (0.49) | 0.37 (0.40) | 0.34 (0.29) | 0.41 (0.33) | 0.62 (0.69) | 0.57 (0.55) | 0.70 (0.62) |
| Fruits (servings /d) | 1.65 (1.43) | 1.40 (1.17) | 1.39 (1.18) | 1.37 (1.13) | 1.16 (0.90) | 1.14 (0.89) | 2.03 (1.66) | 1.46 (1.17) | 1.37 (1.12) |
| Vegetables (servings /d) | 1.80 (1.28) | 1.77 (1.07) | 1.93 (1.17) | 2.72 (1.86) | 2.49 (1.49) | 2.70 (1.53) | 4.26 (2.49) | 3.94 (2.11) | 4.25 (2.13) |
| BMI (kg/m2) | 23.9 (4.1) | 24.2 (4.3) | 24.6 (4.7) | 23.5 (4.5) | 24.5 (5.1) | 25.6 (6.0) | 24.8 (3.0) | 25.5 (3.1) | 26.0 (3.4) |
| Physical Activity (METs-hour/week) | 13.9 (22.6) | 11.6 (18.3) | 10.3 (15.8) | 27.9 (34.9) | 19.3 (24.6) | 17.1 (22.9) | 26.6 (30.0) | 19.6 (22.9) | 17.6 (22.3) |
| Alcohol (g/d) | 6.0 (10.0) | 6.6 (10.7) | 6.7 (11.3) | 3.1 (5.7) | 3.1 (5.9) | 3.1 (6.4) | 8.6 (12.7) | 11.3 (14.8) | 13.8 (17.9) |
| Race | |||||||||
| White adults | 92 (15375) | 94 (15542) | 94 (15983) | 91 (16254) | 95 (17057) | 91 (17020) | 89 (7568) | 90 (7348) | 93 (7943) |
| Black adults | 2 (291) | 1 (150) | 1 (239) | 2 (306) | 1 (216) | 2 (286) | 1 (106) | 1 (59) | 1 (77) |
| Hispanic adults | 1 (172) | 1 (122) | 1 (167) | 2 (387) | 1 (271) | 2 (291) | 0 (41) | 1 (45) | 0 (31) |
| Asian adults | 1 (106) | 1 (96) | 1 (149) | 2 (289) | 1 (192) | 2 (279) | 2 (164) | 2 (132) | 1 (117) |
| Current smoking (cigs/d) | |||||||||
| 1-14 | 8 (1312) | 8 (1297) | 8 (1354) | 5 (864) | 6 (1059) | 5 (1001) | 2 (156) | 3 (209) | 3 (284) |
| 15-24 | 11 (1772) | 12 (1957) | 14 (2319) | 3 (550) | 5 (897) | 6 (1192) | 1 (127) | 4 (284) | 4 (371) |
| >24 | 7 (1110) | 8 (1395) | 10 (1721) | 1 (203) | 2 (348) | 3 (591) | 1 (96) | 2 (185) | 5 (450) |
| Family history of type 2 diabetes | 18 (3032) | 18 (2991) | 19 (3328) | 39 (6898) | 41 (7516) | 44 (8139) | 23 (1972) | 23 (1830) | 23 (2006) |
| History of hypertension | 16 (2667) | 15 (2416) | 15 (2615) | 5 (935) | 6 (1080) | 7 (1352) | 19 (1649) | 19 (1547) | 19 (1670) |
| Multivitamin use | 38 (6328) | 34 (5534) | 32 (5545) | 49 (8709) | 43 (7784) | 41 (7498) | 49 (4166) | 40 (3279) | 37 (3141) |
| Postmenopausal hormone use | 9 (1476) | 8 (1275) | 8 (1321) | 3 (450) | 2 (452) | 3 (487) | / | / | / |
Abbreviations: MET, metabolic equivalent; NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II; HPFS, Health Professionals Follow-up Study
For continuous variables, the values are mean (SD); for categorical variables, the values are in % (n). One serving of unprocessed red meat equals 85 g of pork, beef, or lamb; one serving of processed red meat equals 28 g of bacon or 45 g of hot dog, sausage, salami, bologna, or other processed red meats.
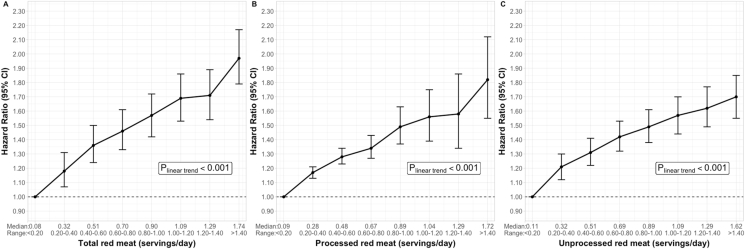
In the 3 cohorts, separately and combined, and adjusting for major confounders, intakes of total red meat, processed red meat, and unprocessed red meat were all associated with a higher risk of T2D (Table 2). Participants in the highest quintile of total red meat, compared with those in the lowest quintile, had a 62% higher risk (HR Q5 vs. Q1 =1.62, 95% CI: 1.53, 1.71, P-trend <0.001) of developing T2D. Comparing the extreme intake quintiles, processed red meat intake was associated with a 51% higher risk of developing T2D (HR Q5 vs. Q1 =1.51, 95% CI: 1.44, 1.58, P-trend <0.001) of developing T2D and unprocessed red meat was associated with a 40% higher risk (HR Q5 vs. Q1 =1.40, 95% CI: 1.33, 1.47, P-trend <0.001). In continuous analysis, every 1 serving/d increment in total red meat was associated with a 1.28 times higher hazard of T2D (95% CI: 1.24, 1.31). Moreover, every 1 serving/d increment of processed red meat was associated with 1.46 times higher hazard of T2D (95% CI: 1.40, 1.53), and that increment of unprocessed red meat was associated with 1.24 times higher hazard of T2D (95% CI: 1.20, 1.29) (Table 2). We also observed approximately linear increases in T2D risks across the red meat intake categories (P-trend <0.001). When compared with people who consumed <0.20 servings of total red meat per day, risk of T2D increased from 1.18 (95% CI: 1.07, 1.31) times among those in 0.20 to 0.40 servings/d category to 1.97 (95% CI: 1.79, 2.17) times among those in ≥1.40 servings/d category. A similar increase in T2D risks was also observed across intake categories of processed red meat and unprocessed red meat (Figure 1, Supplemental Table 3). The test of a possible nonlinear relation between red meats and risk of T2D suggested that the dose-response relation could also be nonlinear but still with a monotonical increase in risk (Supplemental Figure 2).
TABLE 2.
Associations between red meat intake and risk of diabetes in the NHS (n = 84,315), NHS II (n = 90,217), and HPFS (n = 42,163)
| Quintiles of red meat intakes 1 |
P-trend | HR per serving/d (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |||
| Total red meat | |||||||
| Intake (servings/d) 2 | 0.45/0.26/0.27 | 0.75/0.56/0.61 | 1.01/0.79/0.93 | 1.31/1.06/1.30 | 1.86/1.56/1.97 | ||
| Calibrated intake (servings/day) | 0.65/0.49/0.76 | 0.85/0.70/1.11 | 1.00/0.84/1.37 | 1.18/1.00/1.66 | 1.50/1.28/2.16 | ||
| Cases/Person-years | 3187/1091541 | 4015/1093825 | 4676/1096145 | 5168/1098679 | 5715/1103791 | ||
| Model 1 3 | Ref. | 1.29 (1.23, 1.35) | 1.55 (1.48, 1.63) | 1.82 (1.74, 1.91) | 2.37 (2.26, 2.49) | <0.001 | 1.56 (1.52, 1.59) |
| Model 2 4 | Ref. | 1.06 (1.01, 1.11) | 1.13 (1.08, 1.18) | 1.16 (1.10, 1.22) | 1.23 (1.16, 1.30) | <0.001 | 1.12 (1.09, 1.15) |
| Model 3 5 | Ref. | 1.18 (1.12, 1.23) | 1.32 (1.26, 1.38) | 1.42 (1.35, 1.50) | 1.62 (1.53, 1.71) | <0.001 | 1.28 (1.24, 1.31) |
| Processed red meat | |||||||
| Intake (servings/ day) | 0.05/0.01/0.02 | 0.14/0.08/0.12 | 0.22/0.15/0.21 | 0.35/0.25/0.37 | 0.61/0.45/0.71 | ||
| Calibrated intake (servings/day) | 0.13/0.12/0.12 | 0.20/0.18/0.25 | 0.26/0.23/0.36 | 0.33/0.29/0.50 | 0.48/0.41/0.76 | ||
| Cases/Person-years | 3228/1119091 | 4064/1069318 | 4480/1066850 | 5074/1125734 | 5915/1102989 | ||
| Model 1 | Ref. | 1.32 (1.26, 1.38) | 1.49 (1.42, 1.56) | 1.68 (1.61, 1.76) | 2.13 (2.03, 2.22) | <0.001 | 1.93 (1.87, 2.00) |
| Model 2 | Ref. | 1.06 (1.01, 1.11) | 1.07 (1.02, 1.12) | 1.11 (1.06, 1.16) | 1.16 (1.10, 1.22) | <0.001 | 1.21 (1.15, 1.28) |
| Model 3 | Ref. | 1.20 (1.14, 1.26) | 1.27 (1.21, 1.33) | 1.36 (1.30, 1.43) | 1.51 (1.44, 1.58) | <0.001 | 1.46 (1.40, 1.53) |
| Unprocessed red meat | |||||||
| Intake (servings/day) | 0.33/0.19/0.19 | 0.53/0.43/0.43 | 0.74/0.60/0.64 | 0.97/0.80/0.92 | 1.37/1.20/1.39 | ||
| Calibrated intake (servings/day) | 0.49/0.38/0.57 | 0.62/0.51/0.81 | 0.72/0.60/0.97 | 0.84/0.71/1.16 | 1.04/0.90/1.46 | ||
| Cases/Person-years | 3331/1101890 | 4148/1084907 | 4718/1094944 | 5232/1098858 | 5332/1103382 | ||
| Model 1 | Ref. | 1.25 (1.19, 1.31) | 1.41 (1.35, 1.47) | 1.66 (1.59, 1.74) | 1.96 (1.87, 2.06) | <0.001 | 1.58 (1.53, 1.62) |
| Model 2 | Ref. | 1.06 (1.01, 1.11) | 1.08 (1.03, 1.13) | 1.13 (1.08, 1.19) | 1.14 (1.09, 1.21) | <0.001 | 1.10 (1.06, 1.14) |
| Model 3 | Ref. | 1.15 (1.09, 1.20) | 1.21 (1.16, 1.27) | 1.33 (1.27, 1.39) | 1.40 (1.33, 1.47) | <0.001 | 1.24 (1.20, 1.29) |
Abbreviations: NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II; HPFS, Health Professionals Follow-up Study
Dietary intake was cumulative average from the baseline FFQ to the start of each 4-y follow-up interval. One serving of unprocessed red meat equals 85 g of pork, beef, or lamb; one serving of processed red meat equals 28 g of bacon or 45 g of hot dog, sausage, salami, bologna, or other processed red meats.
Medians in the NHS/NHS II/HPFS.
Model 1 was stratified jointly by age in months and calendar time in 2-y groups and adjusted for total energy intake.
Model 2 was additionally adjusted for race/ethnicity (white adults, non-white adults), smoking status (never, past, current: 1-14 cigs/d, current: >15-24 cigs/d, current: >24 cigs/d), alcohol intake (non-alcohol drinker, 0-4.9 grams/d, 5-9.9 grams/d, 10-14.9 grams/d, 15-29.9 grams/d, >30 g/d), physical activity (<3, 3-9, 9-18, 18-27, ≥ 27 METs-h/week), multivitamin use, menopausal status and hormone use (if NHS or NHS II), family history of type 2 diabetes, antihypertensive drug use, cholesterol-lowering drug use, history of hypertension, glycemic index, poultry, fish, egg, total dairy, nuts and legumes, fruits, vegetables, whole grain, and refined grain intakes, socioeconomic status, and BMI (<21, 21-23, 23-25, 25-27, 27-30, 30-33, 33-35, 35-40, ≥40 kg/m2).
Model 3 was adjusted for the covariates in Model 2 except for BMI.
FIGURE 1.
Associations between red meat intakes and risk of diabetes in the NHS, NHS II, and HPFS by categories.
A, Hazard ratios of T2D comparing intakes of total red meat to the lowest intake category. B, Hazard ratios of T2D comparing intakes of processed red meat to the lowest intake category. C, Hazard ratios of T2D comparing intakes of unprocessed red meat to the lowest intake category.
One serving of unprocessed red meat equals 85 g of pork, beef, or lamb; one serving of processed red meat equals 28 g of bacon or 45 g of hot dog, sausage, salami, bologna, or other processed red meats. The hazard ratios and 95% confidence intervals of T2D comparing each intake category with the reference category of <0.20 servings/d were estimated with Cox proportional hazards models stratified jointly by age in months and calendar time in 2-y groups and adjusted for race/ethnicity, smoking status, alcohol intake, physical activity (METs-hours/week), multivitamin use, menopausal status and hormone use (if in NHS or NHS II), family history of T2D, antihypertensive drug use, cholesterol-lowering drug use, baseline history of hypertension, glycemic index, poultry, fish, eggs, total dairy, nuts and legumes, fruits, vegetables, whole grain, and refined grain intakes, and socioeconomic status. Dietary intakes were cumulative averages from the baseline FFQ to the start of each 4-y follow-up interval.
Abbreviations: NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II; HPFS, Health Professionals Follow-up Study
These associations were substantially attenuated after further adjusting for time-varying BMI, a potential mediator. The estimated percentage higher risk of T2D reduced to 12% for every 1 serving/d increment in total red meat (HR per serving/d = 1.12, 95% CI: 1.09, 1.15), 21% for processed red meat (HR per serving/d = 1.21, 95% CI: 1.15, 1.28), and 10% for unprocessed red meat (HR per serving/d = 1.10, 95% CI: 1.06, 1.14) after adjusting for time-varying BMI (Table 2). We also observed attenuation in the associations between red meats and T2D after further adjusting baseline BMI based on the primary model (Supplemental Table 4). Our results showed that, on average, over 50% of the associations between red meat intake and risk of diabetes were statistically accounted for by time-varying BMI (Supplemental Table 5). In the analysis that mutually adjusted for processed and unprocessed red meats, the associations with T2D attenuated but remained strong and statistically significant (Supplemental Table 6). The positive associations between all types of red meat and T2D were consistent across subgroups defined by BMI and baseline hypertension (Supplemental Table 7). However, we observed a higher risk of T2D associated with red meat among people with higher physical activity levels (P-interaction <0.001 for total red meat and processed red meat, P-interaction =0.002 for unprocessed red meat). The associations with T2D risks were stronger among past smokers (P-interaction <0.001 for total red meat, processed red meat, and unprocessed red meat). The associations between red meat consumption and risk of T2D among Black participants (514 cases) were similar to those among White participants (20,546 cases); associations were weaker among Asian (330 cases) and Hispanic (362 cases) participants, but the CIs were wide (Supplemental Table 7).
The associations between red meats and T2D tended to be stronger with a latency of fewer than 12 y, but the positive associations remained statistically significant even when assessing T2D incidence 20 to 24 y after the red meat assessment (Supplemental Table 8). In another analysis, the increased risk of T2D was also mainly associated with red meat intake assessed within 12 y of diagnosis rather than intakes assessed at baseline. Total red meat assessed in the 2 recent follow-up cycles was associated with 1.45 times risk of T2D (HR Q5 vs. Q1 = 1.45, 95% CI: 1.29, 1.63) comparing the extreme quintiles, after additionally controlling for intakes assessed at baseline (Supplemental Table 9).
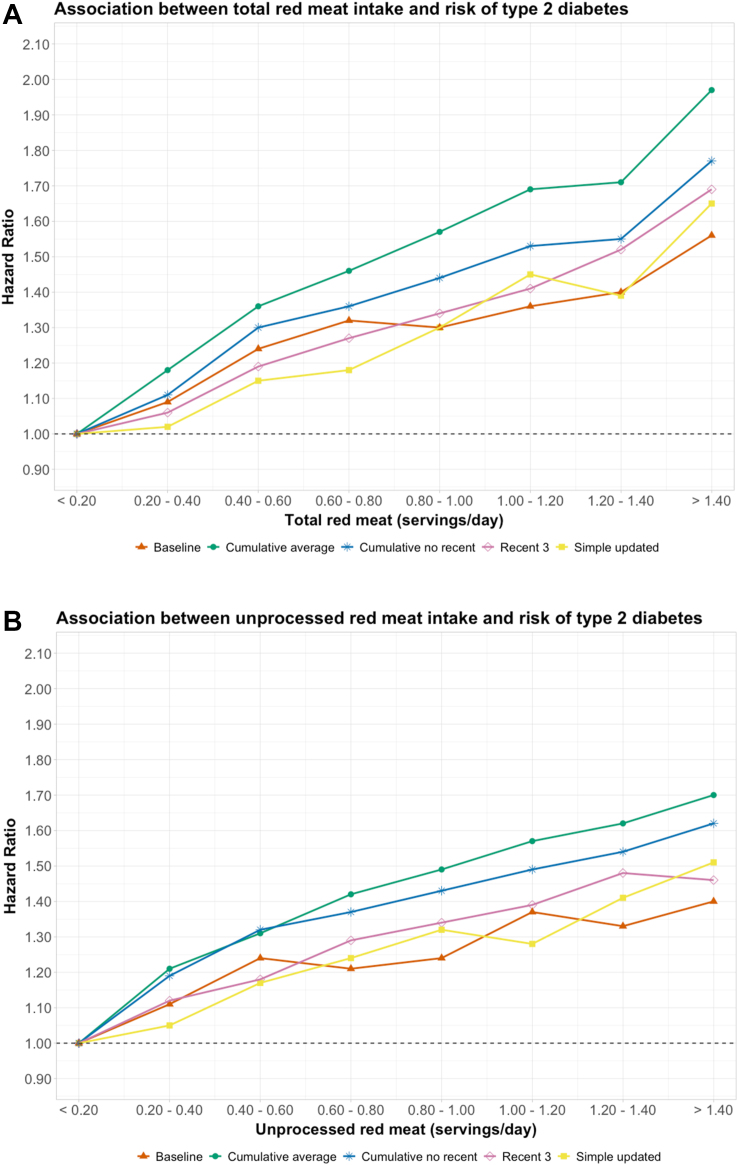
In an analysis comparing different approaches to quantify red meat intakes, the strongest associations with T2D were with cumulative average intake over the follow-up period whereas the weakest associations with T2D were with simple updates and baseline only (Figure 2); for one serving/d of total red meat, the HR was 1.28 (95% CI: 1.24, 1.31) for cumulative average, 1.24 (95% CI: 1.21, 1.27) for the 3 most proximal assessments, 1.23 (95% CI: 1.19, 1.26) after removing the most proximal assessments to minimize reverse causation, 1.20 (95% CI: 1.18, 1.23) for simple updates, and 1.13 (95% CI: 1.11, 1.16) for baseline only (Supplemental Table 10).
FIGURE 2.
Associations between total red meat (A) and unprocessed red meat (B) and risk of diabetes in the NHS, NHS II, and HPFS by categories of intake using different types of dietary assessments.
One serving of unprocessed red meat equals 85 g of pork, beef, or lamb; one serving of processed red meat equals 28 g of bacon or 45 g of hot dog, sausage, salami, bologna, or other processed red meats. The hazard ratios and 95% confidence intervals of T2D comparing each intake category with the reference category of <0.20 servings/d were estimated with Cox proportional hazards models stratified jointly by age in months and calendar time in 2-y groups and adjusted for race/ethnicity, smoking status, alcohol intake, physical activity (METs-hours/week), multivitamin use, menopausal status and hormone use (if in NHS or NHS II), family history of T2D, antihypertensive drug use, cholesterol-lowering drug use, baseline history of hypertension, glycemic index, poultry, fish, eggs, total dairy, nuts and legumes, fruits, vegetables, whole grain, and refined grain intakes, and socioeconomic status. Red meat intakes were calculated as 1) intakes assessed only at baseline (Baseline); 2) cumulative averages of intakes assessed over the follow-up (Cumulative average); 3) cumulative averages of intakes assessed over the follow-up but excluding the most recent 3 assessments prior to T2D diagnosis (Cumulative no recent); 4) cumulative averages of the most recent 3 assessments prior to T2D diagnosis (Recent 3); and 5) simply updated intakes over the follow-up (Simple updated).
Abbreviations: NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II; HPFS, Health Professionals Follow-up Study
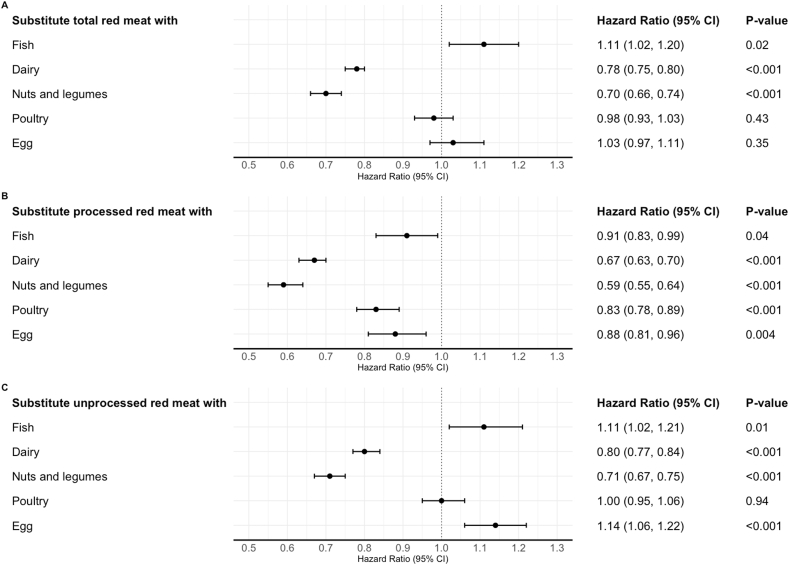
In substitution analyses using 1 serving/d of nuts and legumes as the replacement, replacing 1 serving/d of total red meat was associated with a 30% (HR = 0.70, 95% CI: 0.66, 0.74) lower risk of T2D. Similar associations with comparable magnitude were observed when analyzing nuts and legumes separately as replacements for red meats. The percentage lower risk of T2D associated with substituting 1 serving/d of total dairy for total red meat was 22% (HR = 0.78, 95% CI: 0.75, 0.80) (Figure 3, Supplemental Table 11 and 12).
FIGURE 3.
Associations between substituting one serving of other protein sources for red meat intake and risk of T2D in the NHS, NHS II, and HPFS
One serving of unprocessed red meat equals 85 g of pork, beef, or lamb; one serving of processed red meat equals 28 g of bacon or 45 g of hot dog, sausage, salami, bologna, or other processed red meats. The effect of substituting 1 serving/d of other protein sources for red meats was estimated by including both intakes as continuous variables in the same multivariable Cox model. The model was stratified jointly by age in months and calendar time in 2-y groups and adjusted for race/ethnicity, smoking status, alcohol intake, physical activity (METs-hours/week), multivitamin use, menopausal status and hormone use (if in NHS or NHS II), family history of T2D, antihypertensive drug use, cholesterol-lowering drug use, baseline history of hypertension, glycemic index, socioeconomic status, and dietary covariates intakes (including poultry, fish, eggs, total dairy, nuts and legumes, fruits, vegetables, whole grain, and refined grain) excluding the foods subject for substitutions. The HRs (and 95% confidence intervals [(CIs)]) were estimated using the difference in the estimated coefficients (and their pooled variances).
Abbreviations: NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II; HPFS, Health Professionals Follow-up Study
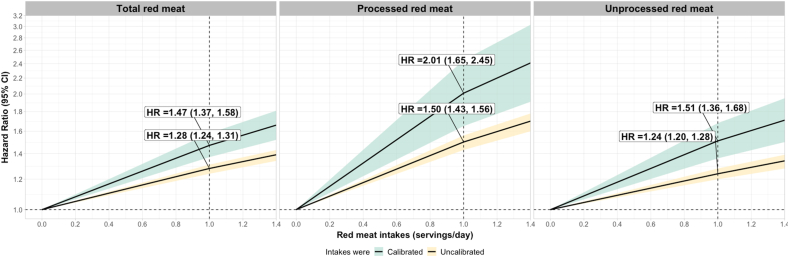
Stronger associations between red meat intakes and T2D risk were observed after calibrating dietary exposures. Before the calibration, a 1-serving intake increment in total red meat was associated with a 28% higher risk of T2D (HR = 1.28, CI: 1.24, 1.31); after the calibration, this was 47% (HR =1.47, CI: 1.37, 1.58). Before the calibration, a 1-serving intake increment in processed red meat was associated with a 50% higher risk of T2D (HR = 1.50, CI: 1.43, 1.56); after the calibration, this was 101% (HR = 2.01, CI: 1.65, 2.45). Before the calibration, a 1-serving intake increment in unprocessed red meat was associated with a 24% higher risk of T2D (HR =1.24, CI: 1.20, 1.28); after the calibration, this was 51% (HR=1.51, CI: 1.36, 1.68) (Figure 4, Supplemental Table 13). Cohort-specific results were presented in Supplemental Tables 14–18.
FIGURE 4.
Associations between every 1 serving/d increase in uncalibrated and calibrated red meat intakes and risk of diabetes in the NHS, NHS II, and HPFS
One serving of unprocessed red meat equals 85 g pork, beef, or lamb; one serving of processed red meat equals 28 g bacon or 45 g hot dog, sausage, salami, bologna, or other processed red meats. We regressed red meats assessed by 7DDRs on intake assessed by FFQs using a two-part regression to account for intakes reported as zeros. The first part of the two-part regression employed logistic regression to handle zero and nonzero data, while the second part utilized linear regression to model nonzero intakes. For the dietary covariates, we fitted intakes measured with 7DDRs and FFQs with linear regressions. We calculated the cumulative averaged calibrated intakes and used Cox proportional hazards models to estimate their associations with T2D. The model was stratified jointly by age in months and calendar time in 2-y groups and adjusted for total energy intake, race/ethnicity (white adults, non-white adults), smoking status (never, past, current: 1-14 cigs/d, current: >15-24 cigs/d, current: >24 cigs/d), physical activity (<3, 3-9, 9-18, 18-27, ≥ 27 METs-h/week), multivitamin use, menopausal status and hormone use (if NHS or NHS II), family history of type 2 diabetes, antihypertensive drug use, cholesterol-lowering drug use, history of hypertension, AHEI-2010 excluding meat component, and socioeconomic status.
Abbreviations: NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II; HPFS, Health Professionals Follow-up Study
Discussion
In this study of 216,695 United States females and males, intakes of red meat, including both processed and unprocessed red meats, were strongly associated with higher risks of T2D with an approximately linear dose-response relationship. These associations remained robust after statistically accounting for BMI, which may be a mediating factor. Replacing total red meat with nuts and legumes, and dairy foods was each associated with a lower risk of T2D. Red meats intake was most strongly associated with risk of T2D when being calculated as the cumulative average of all questionnaire assessments over the 30-y follow-up period. After calibrating dietary assessments, the associations of interest became stronger. Our results confirm previous findings on the relationship between red meat consumption and T2D with additional quantification and information on temporal relationships [4].
Positive associations between red meats and T2D have been observed in other prospective studies [[4], [32], [33], [34]]. Similar to our findings, processed meat intake was more strongly associated with a higher risk of T2D than total red meat intake [[32], [33], [34]]. In the EPIC-InterAct study, which is the next largest diabetes cohort with 12,403 incident cases accumulated by 2007, investigators observed an 18% and 20% higher risk of T2D associated with every 50 grams increase in unprocessed red meat and processed red meat intake, respectively [35]. However, the magnitude of the associations we observed was stronger than those from previous studies [4, 33, 34]. Possible explanations for the difference are that in most studies, diet was assessed only at baseline, which would not capture dietary change and cumulative effects of red meats on T2D development. Our latency analysis supported the hypothesis that red meat’s association with diabetes risk is strongest within 10 to 15 y before T2D diagnosis. Therefore, diabetes risk more than this time after dietary assessment, such as the baseline in most prospective cohorts with long follow-up, will likely be less strongly associated with intake of red meat. Moreover, our findings show that the average of repeated red meat assessments, which accounts for cumulative exposure and reduces the effect of measurement error, has a much stronger association with T2D incidence than a single assessment of diet at baseline. The UK Biobank Study, which also attempted to assess usual meat consumption through measurement error correction, found a slightly weaker association (HR=1.30, CI: 1.20, 1.42) between every 70 grams/d (equivalent to about 1 serving/d) total red meat intake and risk of T2D (9,571 cases) among males and females compared with our calibrated associations [34].
Recent studies of metabolomics signatures of red meat and their associations with T2D further added biological plausibility to the findings from observational studies [36, 37]. In the EPIC-Norfolk study with comprehensive metabolomics profiling, 139 metabolites consisting of lipids, amino acids, xenobiotics, and other unknown metabolites were identified to be associated with red meat consumption. A metabolite score for red meat consumption was then derived and validated in both the observational EPIC-Norfolk study and a randomized controlled crossover trial conducted in France. Every standard deviation increase in this red meat metabolite score was found to be associated with a 17% higher risk of T2D [36]. Metabolite scores for total meat, red meat, and processed red meat were also derived in the PREDIMED study based on 72, 69, and 74 serum metabolites, respectively. The percentage increase in T2D risk associated with every standard deviation higher baseline metabolite score was 25% for total meat, 27% for red meat, and 27% for processed red meat [37].
Multiple biological mechanisms may contribute to the higher risk of T2D among consumers of red meat. Saturated fat, which is high in red meat, can reduce beta cell function and insulin sensitivity [38, 39]. The relatively low content of polyunsaturated fat in red meat could result in an increased risk of T2D since linoleic acid is an agonist of selective peroxisome proliferator-activated receptor (PPAR) [39, 40]. In a meta-analysis of 30 RCTs with short durations, substituting 5% energy from polyunsaturated fat, primarily linoleic acid, for saturated fat reduced insulin resistance (HOMA-IR) by 4.1% [41]. Heme iron, as a strong prooxidant, increases oxidative stress and insulin resistance and impairs beta cell function through its by-product of nitroso compounds [42, 43]. Plasma ferritin level, as an indicator of iron intake and storage, is also associated with total red meat consumption and increased diabetes risk independently [44, 45]. Processed red meats often have a high content of nitrates and their byproducts, which promote endothelial dysfunction and insulin resistance [46]. Elevated glycine utilization, which is related to heme biosynthesis, was observed after red meat intake and was associated with higher diabetes risk [47]. Dietary tryptophan, which is mainly from animal protein sources such as red meats and dairy, and its metabolites were also shown to be associated with increased diabetes risk [48].
Body adiposity, characterized by BMI, has also been proposed as another mediator for the association between red meat and T2D. In US females and males, processed red meat and unprocessed red meat consumption were among the dietary factors having the largest positive associations with weight gain in a 4-y period [49]. An 8-wk randomized trial showed that people consuming plant-based alternative meat, which is soy or pea protein-based, had significantly lower body weight (1 kg) than those consuming animal-based meat [50]. Also, excess adiposity is known to increase T2D risks through the development of insulin resistance, dyslipidemia, and inflammation [51], and weight gain from early to middle adulthood is strongly associated with elevated risk of T2D [52]. Body adiposity could also be a confounder if health awareness leads to both lower red meat consumption and better weight control. Because of the likelihood that weight gain mediates at least part of the association between red meat intake and risk of T2D, we did not adjust for adiposity in the primary analysis; with adjustment for BMI, the positive association was partially attenuated but still highly significant.
A recent meta-analysis concluded that the dose-response relation between unprocessed red meat and T2D is completely uncertain [53]. However, the method was seriously flawed because it was meant to predict a single new study rather than summarize available evidence [54]. Also, this analysis assumed that between-study heterogeneity simply reflects the uncertainty in the true relationship rather than the differences in the designs and qualities of studies, leading to a drastic inflation of the uncertainty interval. Our analysis strongly responds to the authors’ call for rigorous and well-powered research that helps understand and quantify the relationship between red meat consumption and T2D [53].
Because a person’s long-term energy intake is regulated within narrow limits without changes in weight or physical activity [55], reductions in red meat, which is high in fat and protein, will be largely compensated by other sources of energy. Our substitution analyses suggest that among the 5 protein sources we investigated as alternatives, nuts and legumes were associated with the most substantial risk reductions. This finding is consistent with evidence that sources of unsaturated fatty acids and antioxidants have beneficial effects on glycemic control, insulin response, and inflammation [[56], [57], [58]]. Other evidence suggesting neutral or beneficial effects of dairy foods on risks of T2D are consistent with our findings on alternatives to red meats [59, 60].
The current study has multiple strengths. With the availability of repeated dietary assessments in our cohorts over 3 decades, we were not only able to assess the cumulative intake of red meat but also to compare the impacts of different approaches for describing dietary exposure, including using only the baseline diet. The large number of incident cases of T2D provided high statistical precision, and the availability of calibration data allowed adjustment for measurement error. Our investigation of the latency period would not be possible in randomized trials that typically have very short follow-up. The responses of markers reflecting beta cell function or glycemic control following interventions with a diet high in red meat may not be observable when they are assessed only postprandially or within up to 16 wk of trial follow-up [10, 38].
Our study was also subject to limitations. Our findings may have limited generalizability because all participants were health professionals. However, it is reasonable to extrapolate our conclusions to the general population as people with different occupations are biologically similar. Conducting analysis in health professionals also reduces potential confounding by socioeconomic status. The almost unchanged effect estimates in our sensitivity analysis adjusting socioeconomic status showed that residual confounding due to socioeconomic status is likely to be limited. Our study population consists of more than 90% Caucasians. After pooling data from all 3 cohorts, we had sufficient numbers of cases to evaluate associations, at least among Black participants, and found associations similar to those among White cohort members. Somewhat weaker associations were seen among Asian and Hispanic participants, which may be due to insufficient power or different ways of consuming red meat. Our analyses did not account for food preparation methods as that was not assessed with the FFQs. Hazardous chemicals such as advanced glycation end products and fatty acid isomers could be produced when deep frying or grilling fish and poultry and, therefore, diminish their health benefits when substituting red meats in diet [61]. Finally, we cannot exclude the possibility of residual confounding due to the observational nature of our study.
In conclusion, we found that a higher intake of total red meat, processed red meat, and unprocessed red meat was strongly associated with a higher risk of T2D. Replacing 1 serving of total dairy and nuts and legumes for all types of red meat was associated with lower risks of T2D. Associations were strongest using the repeated assessment of diet over the follow-up period and were further strengthened with calibration to account for measurement error. Our study supports current dietary recommendations for limiting the consumption of red meat and emphasizes the importance of different alternative sources of protein for T2D prevention.
Author contributions
The authors responsibilities were as follows—XG, BR, and WCW designed research; WCW conducted research; XG analyzed data; all authors wrote the paper. WCW had primary responsibility for the final content. All authors read and approved the final manuscript. The authors assume full responsibility for analyses and interpretation of these data.
Conflict of Interest
All authors have no conflicts of interest.
Funding
The Nurses’ Health Study (NHS), NHS II, and Health Professionals Follow-up Study are supported by the National Institute of Health (grants UM1 CA186107, U01 CA176726, and U01 CA167552). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data availability
Data described in the manuscript, code book, and analytic code will be made available upon request, pending a letter of intent and a research proposal.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2023.08.021.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zheng Y., Ley S.H., Hu F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018;14:88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 2.Khan M.A.B., Hashim M.J., King J.K., Govender R.D., Mustafa H., Al Kaabi J. Epidemiology of type 2 diabetes - global burden of disease and forecasted trends. J. Epidemiol. Glob. Health. 2020;10:107–111. doi: 10.2991/jegh.k.191028.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neuenschwander M., Ballon A., Weber K.S., Norat T., Aune D., Schwingshackl L., et al. Role of diet in type 2 diabetes incidence: umbrella review of meta-analyses of prospective observational studies. BMJ. 2019;366:l2368. doi: 10.1136/bmj.l2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan A., Sun Q., Bernstein A.M., Schulze M.B., Manson J.E., Willett W.C., et al. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am. J. Clin. Nutr. 2011;94:1088–1096. doi: 10.3945/ajcn.111.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wurtz A.M.L., Jakobsen M.U., Bertoia M.L., Hou T., Schmidt E.B., Willett W.C., et al. Replacing the consumption of red meat with other major dietary protein sources and risk of type 2 diabetes mellitus: a prospective cohort study. Am. J. Clin. Nutr. 2021;113:612–621. doi: 10.1093/ajcn/nqaa284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibsen D.B., Jakobsen M.U., Halkjaer J., Tjonneland A., Kilpelainen T.O., Parner E.T., et al. Replacing red meat with other nonmeat food sources of protein is associated with a reduced risk of type 2 diabetes in a Danish cohort of middle-aged adults. J. Nutr. 2021;151:1241–1248. doi: 10.1093/jn/nxaa448. [DOI] [PubMed] [Google Scholar]
- 7.Ibsen D.B., Steur M., Imamura F., Overvad K., Schulze M.B., Bendinelli B., et al. Replacement of red and processed meat with other food sources of protein and the risk of type 2 diabetes in European populations: the EPIC-interact study. Diabetes Care. 2020;43:2660–2667. doi: 10.2337/dc20-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ibsen D.B., Warberg C.K., Wurtz A.M.L., Overvad K., Dahm C.C. Substitution of red meat with poultry or fish and risk of type 2 diabetes: a Danish cohort study. Eur. J. Nutr. 2019;58:2705–2712. doi: 10.1007/s00394-018-1820-0. [DOI] [PubMed] [Google Scholar]
- 9.Gardner C. "Instead of what," and repeated 4-year interval change regarding red meat and T2D: increasing causal inference in nutritional epidemiology through methodological advances. Am. J. Clin. Nutr. 2021;113:497–498. doi: 10.1093/ajcn/nqaa385. [DOI] [PubMed] [Google Scholar]
- 10.O'Connor L.E., Kim J.E., Clark C.M., Zhu W., Campbell W.W. Effects of total red meat intake on glycemic control and inflammatory biomarkers: a meta-analysis of randomized controlled trials. Adv. Nutr. 2021;12:115–127. doi: 10.1093/advances/nmaa096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders L.M., Wilcox M.L., Maki K.C. Red meat consumption and risk factors for type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2023;77:156–165. doi: 10.1038/s41430-022-01150-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian F., Riddle M.C., Wylie-Rosett J., Hu F.B. Red and processed meats and health risks: how strong is the evidence? Diabetes Care. 2020;43:265–271. doi: 10.2337/dci19-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vernooij R.W.M., Zeraatkar D., Han M.A., El Dib R., Zworth M., Milio K., et al. Patterns of red and processed meat consumption and risk for cardiometabolic and cancer outcomes: a systematic review and meta-analysis of cohort studies. Ann. Intern. Med. 2019;171:732–741. doi: 10.7326/M19-1583. [DOI] [PubMed] [Google Scholar]
- 14.Yuan C., Spiegelman D., Rimm E.B., Rosner B.A., Stampfer M.J., Barnett J.B., et al. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am. J. Epidemiol. 2017;185:570–584. doi: 10.1093/aje/kww104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Shaar L., Yuan C., Rosner B., Dean S.B., Ivey K.L., Clowry C.M., et al. Reproducibility and validity of a semiquantitative food frequency questionnaire in men assessed by multiple methods. Am. J. Epidemiol. 2021;190:1122–1132. doi: 10.1093/aje/kwaa280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Health Professionals Follow-up Study. Internet: https://sites.sph.harvard.edu/hpfs/ (accessed Sep 10 2022).
- 17.Nurses' Health Study. Internet: https://nurseshealthstudy.org/ (accessed Sep 10 2022).
- 18.Feskanich D., Rimm E.B., Giovannucci E.L., Colditz G.A., Stampfer M.J., Litin L.B., et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J. Am. Diet. Assoc. 1993;93:790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 19.Salvini S., Hunter D.J., Sampson L., Stampfer M.J., Colditz G.A., Rosner B., et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int. J. Epidemiol. 1989;18:858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 20.Harvard T.H. Chan School of Public Health Nutrition Department's Food Composition Table. https://regepi.bwh.harvard.edu/health/nutrition/ Internet:
- 21.Hart J.E., Liao X., Hong B., Puett R.C., Yanosky J.D., Suh H., et al. The association of long-term exposure to PM2.5 on all-cause mortality in the nurses' health study and the impact of measurement-error correction. Environ. Health. 2015;14:38. doi: 10.1186/s12940-015-0027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 23.Hu F.B., Leitzmann M.F., Stampfer M.J., Colditz G.A., Willett W.C., Rimm E.B. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch. Intern. Med. 2001;16:1542–1548. doi: 10.1001/archinte.161.12.1542. [DOI] [PubMed] [Google Scholar]
- 24.Manson J.E., Rimm E.B., Stampfer M.J., Colditz G.A., Willett W.C., Krolewski A.S., et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991;338:774–778. doi: 10.1016/0140-6736(91)90664-b. [DOI] [PubMed] [Google Scholar]
- 25.Gu X., Wang D.D., Sampson L., Barnett J.B., Rimm E.B., Stampfer M.J., et al. Validity and reproducibility of a semiquantitative food frequency questionnaire for measuring intakes of foods and food groups. Am. J. Epidemiol. 2023 doi: 10.1093/aje/kwad170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durrleman S., Simon R. Flexible regression models with cubic splines. Stat. Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 27.Bernstein A.M., Sun Q., Hu F.B., Stampfer M.J., Manson J.E., Willett W.,C. Major dietary protein sources and risk of coronary heart disease in women. Circulation. 2010;122:876–883. doi: 10.1161/CIRCULATIONAHA.109.915165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halton T.L., Willett W.C., Liu S., Manson J.E., Stampfer M.J., Hu F.B. Potato and french fry consumption and risk of type 2 diabetes in women. Am. J. Clin. Nutr. 2006;83:284–290. doi: 10.1093/ajcn/83.2.284. [DOI] [PubMed] [Google Scholar]
- 29.Spiegelman D., McDermott A., Rosner B. Regression calibration method for correcting measurement-error bias in nutritional epidemiology. Am. J. Clin. Nutr. 1997;65:1179S–1186S. doi: 10.1093/ajcn/65.4.1179S. [DOI] [PubMed] [Google Scholar]
- 30.Duan N., Manning W.G., Morris C.N., Newhouse J.P. Choosing between the sample-selection model and the multi-part model. J. Bus. Econ. Stat. 1984;2:283–289. [Google Scholar]
- 31.Smith-Warner S.A., Spiegelman D., Ritz J., Albanes D., Beeson W.L., Bernstein L., et al. Methods for pooling results of epidemiologic studies: the pooling project of prospective studies of diet and cancer. Am. J. Epidemiol. 2006;163:1053–1064. doi: 10.1093/aje/kwj127. [DOI] [PubMed] [Google Scholar]
- 32.Feskens E.J., Sluik D., van Woudenbergh G.J. Meat consumption, diabetes, and its complications. Curr. Diab. Rep. 2013;13:298–306. doi: 10.1007/s11892-013-0365-0. [DOI] [PubMed] [Google Scholar]
- 33.Schwingshackl L., Hoffmann G., Lampousi A.M., Knuppel S., Iqbal K., Schwedhelm C., et al. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017;32:363–375. doi: 10.1007/s10654-017-0246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papier K., Fensom G.K., Knuppel A., Appleby P.N., Tong T.Y.N., Schmidt J.A., et al. Meat consumption and risk of 25 common conditions: outcome-wide analyses in 475,000 men and women in the UK Biobank study. BMC Med. 2021;19:53. doi: 10.1186/s12916-021-01922-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Consorcium InterAct, Bendinelli B., Palli D., Masala G., Sharp S.J., Schulze M.B., et al. Association between dietary meat consumption and incident type 2 diabetes: the EPIC-InterAct study. Diabetologia. 2013;56:47–59. doi: 10.1007/s00125-012-2718-7. [DOI] [PubMed] [Google Scholar]
- 36.Li C., Imamura F., Wedekind R., Stewart I.D., Pietzner M., Wheeler E., et al. Development and validation of a metabolite score for red meat intake: an observational cohort study and randomized controlled dietary intervention. Am. J. Clin. Nutr. 2022;116:511–522. doi: 10.1093/ajcn/nqac094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-Gavilan J., Nishi S.K., Paz-Graniel I., Guasch-Ferre M., Razquin C., Clish C.B., et al. Plasma metabolite profiles associated with the amount and source of meat and fish consumption and the risk of type 2 diabetes. Mol. Nutr. Food Res. 2022;66 doi: 10.1002/mnfr.202200145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez S., Bermudez B., Pacheco Y.M., Villar J., Abia R., Muriana F.J. Distinctive postprandial modulation of beta cell function and insulin sensitivity by dietary fats: monounsaturated compared with saturated fatty acids. Am. J. Clin. Nutr. 2008;88:638–644. doi: 10.1093/ajcn/88.3.638. [DOI] [PubMed] [Google Scholar]
- 39.Riserus U. Fatty acids and insulin sensitivity. Curr. Opin. Clin. Nutr. Metab. Care. 2008;11:100–105. doi: 10.1097/MCO.0b013e3282f52708. [DOI] [PubMed] [Google Scholar]
- 40.Hwang D. Fatty acids and immune responses--a new perspective in searching for clues to mechanism. Annu. Rev. Nutr. 2000;20:431–456. doi: 10.1146/annurev.nutr.20.1.431. [DOI] [PubMed] [Google Scholar]
- 41.Imamura F., Micha R., Wu J.H., de Oliveira Otto M.C., Otite F.O., Abioye A.I., et al. Effects of saturated fat, polyunsaturated fat, monounsaturated fat, and carbohydrate on glucose-insulin homeostasis: a systematic review and meta-analysis of randomised controlled feeding trials. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Oliveira Otto M.C., Alonso A., Lee D.H., Delclos G.L., Bertoni A.G., Jiang R., et al. Dietary intakes of zinc and heme iron from red meat, but not from other sources, are associated with greater risk of metabolic syndrome and cardiovascular disease. J. Nutr. 2012;142:526–533. doi: 10.3945/jn.111.149781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Misra R., Balagopal P., Raj S., Patel T.,G. Red meat consumption (heme iron intake) and risk for diabetes and comorbidities? Curr. Diab. Rep. 2018;18:100. doi: 10.1007/s11892-018-1071-8. [DOI] [PubMed] [Google Scholar]
- 44.Ley S.H., Sun Q., Willett W.C., Eliassen A.H., Wu K., Pan A., et al. Associations between red meat intake and biomarkers of inflammation and glucose metabolism in women. Am. J. Clin. Nutr. 2014;99:352–360. doi: 10.3945/ajcn.113.075663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swaminathan S., Fonseca V.A., Alam M.G., Shah S.V. The role of iron in diabetes and its complications. Diabetes Care. 2007;30:1926–1933. doi: 10.2337/dc06-2625. [DOI] [PubMed] [Google Scholar]
- 46.Micha R., Michas G., Mozaffarian D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes--an updated review of the evidence. Curr. Atheroscler. Rep. 2012;`14:515–524. doi: 10.1007/s11883-012-0282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wittenbecher C., Muhlenbruch K., Kroger J., Jacobs S., Kuxhaus O., Floegel A., et al. Amino acids, lipid metabolites, and ferritin as potential mediators linking red meat consumption to type 2 diabetes. Am. J. Clin. Nutr. 2015;101:1241–1250. doi: 10.3945/ajcn.114.099150. [DOI] [PubMed] [Google Scholar]
- 48.Yu E., Papandreou C., Ruiz-Canela M., Guasch-Ferre M., Clish C.B., Dennis C., et al. Association of tryptophan metabolites with incident type 2 diabetes in the PREDIMED trial: a case-cohort study. Clin. Chem. 2018;64:1211–1220. doi: 10.1373/clinchem.2018.288720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mozaffarian D., Hao T., Rimm E.B., Willett W.C., Hu F.B. Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med. 2011;364:2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crimarco A., Springfield S., Petlura C., Streaty T., Cunanan K., Lee J., et al. A randomized crossover trial on the effect of plant-based compared with animal-based meat on trimethylamine-N-oxide and cardiovascular disease risk factors in generally healthy adults: study with appetizing plantfood-meat eating alternative trial (SWAP-MEAT) Am. J. Clin. Nutr. 2020;112:1188–1199. doi: 10.1093/ajcn/nqaa203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu F. Oxford University Press; 2008. Obesity epidemiology. [Google Scholar]
- 52.Zheng Y., Manson J.E., Yuan C., Liang M.H., Grodstein F., Stampfer M.J., et al. Associations of weight gain from early to middle adulthood with major health outcomes later in life. JAMA. 2017;318:255–269. doi: 10.1001/jama.2017.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lescinsky H., Afshin A., Ashbaugh C., Bisignano C., Brauer M., Ferrara G., et al. Health effects associated with consumption of unprocessed red meat: a burden of proof study. Nat. Med. 2022;28:2075–2082. doi: 10.1038/s41591-022-01968-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glenn A.J., Gu X., Hu F.B., Wang M., Willett W.C. Concerns about the burden of proof studies. Nat. Med. 2023;29:823–825. doi: 10.1038/s41591-023-02294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willett W., Willett W. Nutritional Epidemiology. Edtion ed. Oxford University Press; 2012. 260Implications of Total Energy Intake for Epidemiologic Analyses; p. 0. [Google Scholar]
- 56.Rajaram S., Sabate J. Nuts, body weight and insulin resistance. Br. J. Nutr. 2006;96:S79–86. doi: 10.1017/bjn20061867. [DOI] [PubMed] [Google Scholar]
- 57.Casas-Agustench P., Lopez-Uriarte P., Bullo M., Ros E., Cabre-Vila J.J., Salas-Salvado J. Effects of one serving of mixed nuts on serum lipids, insulin resistance and inflammatory markers in patients with the metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2011;21:126–135. doi: 10.1016/j.numecd.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 58.Bielefeld D., Grafenauer S., Rangan A. the effects of legume consumption on markers of glycaemic control in individuals with and without diabetes mellitus: a systematic literature review of randomised controlled trials. Nutrients. 2020;12:2123. doi: 10.3390/nu12072123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alvarez-Bueno C., Cavero-Redondo I., Martinez-Vizcaino V., Sotos-Prieto M., Ruiz J.R., Gil A. Effects of milk and dairy product consumption on type 2 diabetes: overview of systematic reviews and meta-analyses. Adv. Nutr. 2019;10:S154–S163. doi: 10.1093/advances/nmy107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133:187–225. doi: 10.1161/CIRCULATIONAHA.115.018585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu G., Zong G., Wu K., Hu Y., Li Y., Willett W.C., et al. Meat cooking methods and risk of type 2 diabetes: results from three prospective cohort studies. Diabetes Care. 2018;41:1049–1060. doi: 10.2337/dc17-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request, pending a letter of intent and a research proposal.