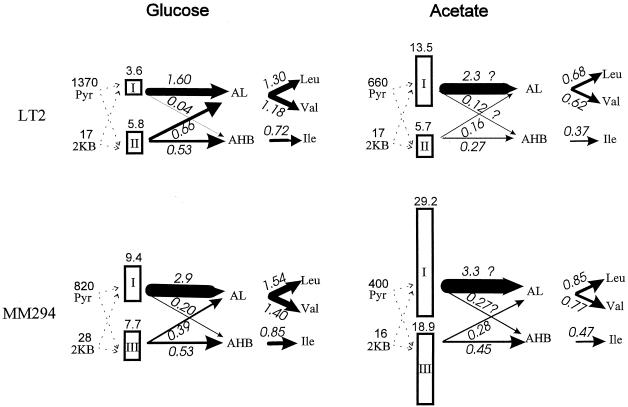
FIG. 3.
Estimated fluxes in the BCAA biosynthetic pathway. Fluxes estimated for S. typhimurium LT2 and E. coli K-12 MM294 in glucose and in acetate minimal media are given in units of millimolar minute−1 over the flux arrows, which are drawn with thickness proportional to flux. Fluxes through the AHAS isozymes to the two acetohydroxy acids (acetolactate [AL] and acetohydroxybutyrate [AHB]) were calculated from the intracellular pyruvate and 2-ketobutyrate (2KB) micromolar concentrations (at left in each portion of the figure [from Table 4]) and from the measured activity of each isozyme (Vmax in units of flux of millimolar minute−1, calculated from the specific activities shown in Table 3 as described in Materials and Methods). Fluxes were calculated from V = Vmax[Pyr]/(Km + [Pyr]), where the Km values are 1.64, 5.3, and 6 mM for AHAS I, II, and III, respectively. Fluxes in acetate medium were approximated for AHAS I or III by assuming that Km(app) = Km(1 + 100 μM/Ki), where the values of Ki, the inhibition constant for glyoxylate, are 140 and 150 μM, respectively, for isozymes I and III. For isozyme II, we used the phenomenological relationship shown in Fig. 2B. The partitioning of flux to the two products, AL and AHB, was calculated from VAHB/VAL = R([KB]/[Pyr]), where the R values are 2, 65, and 40 for AHAS I, II, and III, respectively (29). The required (minimal) flux, J, to each amino acid was calculated from the doubling time of the cells, τ (Table 2), the total amino acid content, A (320, 350, and 190 μmol of Val, Leu, and Ile g [dry weight]−1, respectively), and the assumption that 1 g (dry weight) of cells contains 2.4 ml of intracellular water (50, 58): J = (ln2/τ) × (A/2.4).