Abstract
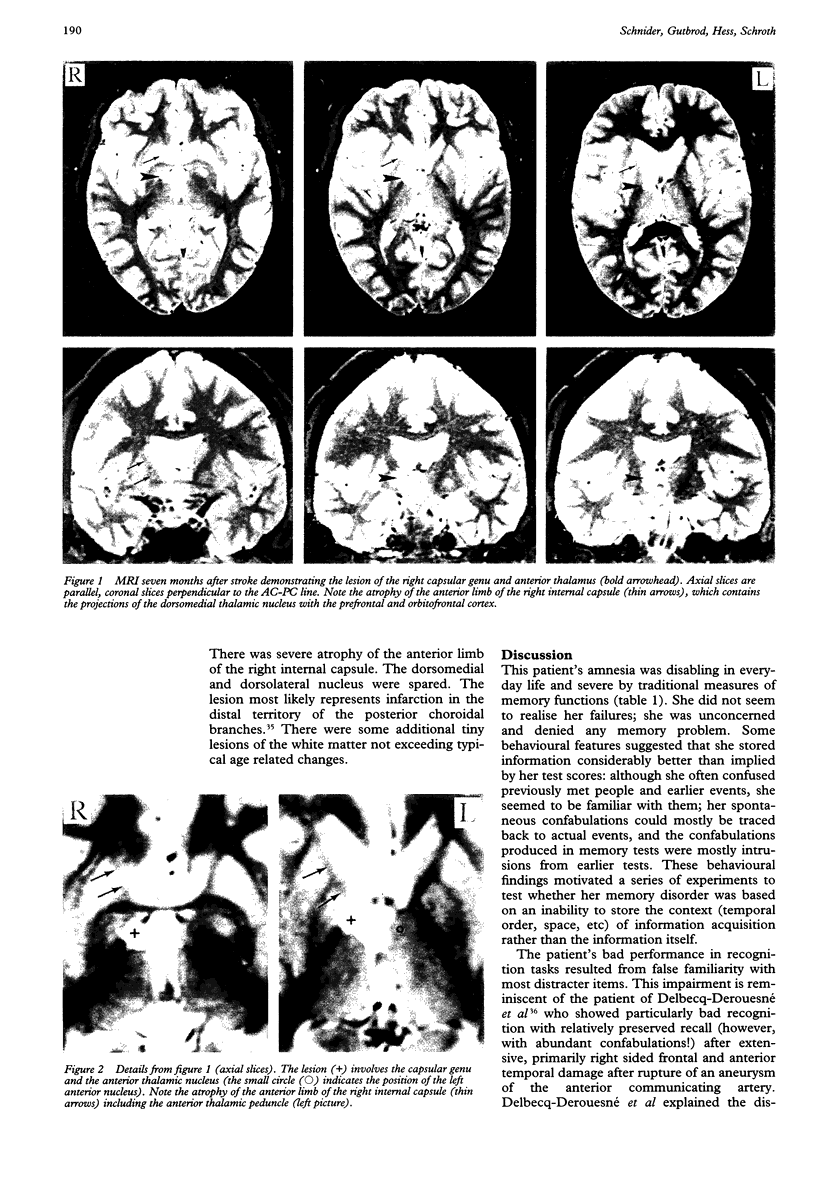
OBJECTIVE--To explore the mechanism of an amnesia marked by confabulations and lack of insight in a patient with an infarct of the right inferior capsular genu. The confabulations could mostly be traced back to earlier events, indicating that the memory disorder ensued from an inability to store the temporal and spatial context of information acquisition rather than a failure to store new information. METHODS--To test the patient's ability to store the context of information acquisition, two experiments were composed in which she was asked to decide when or where she had learned the words from two word lists presented at different points in time or in different rooms. To test her ability to store new information, two continuous recognition tests with novel non-words and nonsense designs were used. Recognition of these stimuli was assumed to be independent of the context of acquisition because the patient could not have an a priori sense of familiarity with them. RESULTS--The patient performed at chance in the experiments probing knowledge of the context of information acquisition, although she recognised the presented words almost as well as the controls. By contrast, her performance was normal in the recognition tests with non-words and nonsense designs. CONCLUSION--These findings indicate that the patient's amnesia was based on an inability to store the context of information acquisition rather than the information itself. Based on an analysis of her lesion, which disconnected the thalamus from the orbitofrontal cortex and the amygdala, and considering the similarities between her disorder, Wernicke-Korsakoff syndrome, and the amnesia after orbitofrontal lesions, it is proposed that contextual amnesia results from interruption of the loop connecting the amygdala, the dorsomedial nucleus, and the orbitofrontal cortex.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggleton J. P., Sahgal A. The contribution of the anterior thalamic nuclei to anterograde amnesia. Neuropsychologia. 1993 Oct;31(10):1001–1019. doi: 10.1016/0028-3932(93)90029-y. [DOI] [PubMed] [Google Scholar]
- Alexander M. P., Freedman M. Amnesia after anterior communicating artery aneurysm rupture. Neurology. 1984 Jun;34(6):752–757. doi: 10.1212/wnl.34.6.752. [DOI] [PubMed] [Google Scholar]
- Damasio A. R., Graff-Radford N. R., Eslinger P. J., Damasio H., Kassell N. Amnesia following basal forebrain lesions. Arch Neurol. 1985 Mar;42(3):263–271. doi: 10.1001/archneur.1985.04060030081013. [DOI] [PubMed] [Google Scholar]
- DeLuca J., Cicerone K. D. Confabulation following aneurysm of the anterior communicating artery. Cortex. 1991 Sep;27(3):417–423. doi: 10.1016/s0010-9452(13)80036-6. [DOI] [PubMed] [Google Scholar]
- DeLuca J., Diamond B. J. Aneurysm of the anterior communicating artery: a review of neuroanatomical and neuropsychological sequelae. J Clin Exp Neuropsychol. 1995 Feb;17(1):100–121. doi: 10.1080/13803399508406586. [DOI] [PubMed] [Google Scholar]
- DeLuca J. Predicting neurobehavioral patterns following anterior communicating artery aneurysm. Cortex. 1993 Dec;29(4):639–647. doi: 10.1016/s0010-9452(13)80287-0. [DOI] [PubMed] [Google Scholar]
- Delbecq-Derouesné J., Beauvois M. F., Shallice T. Preserved recall versus impaired recognition. A case study. Brain. 1990 Aug;113(Pt 4):1045–1074. doi: 10.1093/brain/113.4.1045. [DOI] [PubMed] [Google Scholar]
- Foglia P., Perini M., Vanzulli F. Pure amnesia in a case of right thalamic lesion. Ital J Neurol Sci. 1991 Apr;12(2):211–213. doi: 10.1007/BF02337036. [DOI] [PubMed] [Google Scholar]
- Gentilini M., De Renzi E., Crisi G. Bilateral paramedian thalamic artery infarcts: report of eight cases. J Neurol Neurosurg Psychiatry. 1987 Jul;50(7):900–909. doi: 10.1136/jnnp.50.7.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Radford N. R., Tranel D., Van Hoesen G. W., Brandt J. P. Diencephalic amnesia. Brain. 1990 Feb;113(Pt 1):1–25. doi: 10.1093/brain/113.1.1. [DOI] [PubMed] [Google Scholar]
- Guberman A., Stuss D. The syndrome of bilateral paramedian thalamic infarction. Neurology. 1983 May;33(5):540–546. doi: 10.1212/wnl.33.5.540. [DOI] [PubMed] [Google Scholar]
- Hodges J. R., McCarthy R. A. Autobiographical amnesia resulting from bilateral paramedian thalamic infarction. A case study in cognitive neurobiology. Brain. 1993 Aug;116(Pt 4):921–940. doi: 10.1093/brain/116.4.921. [DOI] [PubMed] [Google Scholar]
- Hunkin N. M., Parkin A. J. Recency judgements in Wernicke-Korsakoff and post-encephalitic amnesia: influences of proactive interference and retention interval. Cortex. 1993 Sep;29(3):485–499. doi: 10.1016/s0010-9452(13)80255-9. [DOI] [PubMed] [Google Scholar]
- Irle E., Wowra B., Kunert H. J., Hampl J., Kunze S. Memory disturbances following anterior communicating artery rupture. Ann Neurol. 1992 May;31(5):473–480. doi: 10.1002/ana.410310503. [DOI] [PubMed] [Google Scholar]
- Kapur N., Coughlan A. K. Confabulation and frontal lobe dysfunction. J Neurol Neurosurg Psychiatry. 1980 May;43(5):461–463. doi: 10.1136/jnnp.43.5.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B., Taylor L. Affective behavior in patients with localized cortical excisions: role of lesion site and side. Science. 1981 Oct 2;214(4516):89–91. doi: 10.1126/science.7280683. [DOI] [PubMed] [Google Scholar]
- Kopelman M. D. Two types of confabulation. J Neurol Neurosurg Psychiatry. 1987 Nov;50(11):1482–1487. doi: 10.1136/jnnp.50.11.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman M. D., Wilson B. A., Baddeley A. D. The autobiographical memory interview: a new assessment of autobiographical and personal semantic memory in amnesic patients. J Clin Exp Neuropsychol. 1989 Oct;11(5):724–744. doi: 10.1080/01688638908400928. [DOI] [PubMed] [Google Scholar]
- Markowitsch H. J., von Cramon D. Y., Hofmann E., Sick C. D., Kinzler P. Verbal memory deterioration after unilateral infarct of the internal capsule in an adolescent. Cortex. 1990 Dec;26(4):597–609. doi: 10.1016/s0010-9452(13)80309-7. [DOI] [PubMed] [Google Scholar]
- Markowitsch H. J., von Cramon D. Y., Schuri U. Mnestic performance profile of a bilateral diencephalic infarct patient with preserved intelligence and severe amnesic disturbances. J Clin Exp Neuropsychol. 1993 Sep;15(5):627–652. doi: 10.1080/01688639308402586. [DOI] [PubMed] [Google Scholar]
- Mercer B., Wapner W., Gardner H., Benson D. F. A study of confabulation. Arch Neurol. 1977 Jul;34(7):429–433. doi: 10.1001/archneur.1977.00500190063009. [DOI] [PubMed] [Google Scholar]
- Milner B., Corsi P., Leonard G. Frontal-lobe contribution to recency judgements. Neuropsychologia. 1991;29(6):601–618. doi: 10.1016/0028-3932(91)90013-x. [DOI] [PubMed] [Google Scholar]
- Mishkin M. A memory system in the monkey. Philos Trans R Soc Lond B Biol Sci. 1982 Jun 25;298(1089):83–95. doi: 10.1098/rstb.1982.0074. [DOI] [PubMed] [Google Scholar]
- Mishkin M. Memory in monkeys severely impaired by combined but not by separate removal of amygdala and hippocampus. Nature. 1978 May 25;273(5660):297–298. doi: 10.1038/273297a0. [DOI] [PubMed] [Google Scholar]
- Mufson E. J., Pandya D. N. Some observations on the course and composition of the cingulum bundle in the rhesus monkey. J Comp Neurol. 1984 May 1;225(1):31–43. doi: 10.1002/cne.902250105. [DOI] [PubMed] [Google Scholar]
- Parkin A. J., Rees J. E., Hunkin N. M., Rose P. E. Impairment of memory following discrete thalamic infarction. Neuropsychologia. 1994 Jan;32(1):39–51. doi: 10.1016/0028-3932(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Plets C., De Reuck J., Vander Eecken H., Van den Bergh R. The vascularization of the human thalamus. Acta Neurol Belg. 1970;70(6):687–770. [PubMed] [Google Scholar]
- Raisman G., Cowan W. M., Powell T. P. An experimental analysis of the efferent projection of the hippocampus. Brain. 1966 Mar;89(1):83–108. doi: 10.1093/brain/89.1.83. [DOI] [PubMed] [Google Scholar]
- Regard M., Strauss E., Knapp P. Children's production on verbal and non-verbal fluency tasks. Percept Mot Skills. 1982 Dec;55(3 Pt 1):839–844. doi: 10.2466/pms.1982.55.3.839. [DOI] [PubMed] [Google Scholar]
- SCOVILLE W. B., MILNER B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957 Feb;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnider A., Bassetti C., Schnider A., Gutbrod K., Ozdoba C. Very severe amnesia with acute onset after isolated hippocampal damage due to systemic lupus erythematosus. J Neurol Neurosurg Psychiatry. 1995 Dec;59(6):644–646. doi: 10.1136/jnnp.59.6.644-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnider A., Gutbrod K., Hess C. W. Motion imagery in Parkinson's disease. Brain. 1995 Apr;118(Pt 2):485–493. doi: 10.1093/brain/118.2.485. [DOI] [PubMed] [Google Scholar]
- Shapiro B. E., Alexander M. P., Gardner H., Mercer B. Mechanisms of confabulation. Neurology. 1981 Sep;31(9):1070–1076. doi: 10.1212/wnl.31.9.1070. [DOI] [PubMed] [Google Scholar]
- Shimamura A. P., Squire L. R. A neuropsychological study of fact memory and source amnesia. J Exp Psychol Learn Mem Cogn. 1987 Jul;13(3):464–473. doi: 10.1037//0278-7393.13.3.464. [DOI] [PubMed] [Google Scholar]
- Shoqeirat M. A., Mayes A. R. Disproportionate incidental spatial-memory and recall deficits in amnesia. Neuropsychologia. 1991;29(8):749–769. doi: 10.1016/0028-3932(91)90070-o. [DOI] [PubMed] [Google Scholar]
- Sillito A. M., Jones H. E., Gerstein G. L., West D. C. Feature-linked synchronization of thalamic relay cell firing induced by feedback from the visual cortex. Nature. 1994 Jun 9;369(6480):479–482. doi: 10.1038/369479a0. [DOI] [PubMed] [Google Scholar]
- Snodgrass J. G., Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol Hum Learn. 1980 Mar;6(2):174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Stuss D. T., Alexander M. P., Lieberman A., Levine H. An extraordinary form of confabulation. Neurology. 1978 Nov;28(11):1166–1172. doi: 10.1212/wnl.28.11.1166. [DOI] [PubMed] [Google Scholar]
- Tatemichi T. K., Desmond D. W., Prohovnik I., Cross D. T., Gropen T. I., Mohr J. P., Stern Y. Confusion and memory loss from capsular genu infarction: a thalamocortical disconnection syndrome? Neurology. 1992 Oct;42(10):1966–1979. doi: 10.1212/wnl.42.10.1966. [DOI] [PubMed] [Google Scholar]
- Tranel D., Hyman B. T. Neuropsychological correlates of bilateral amygdala damage. Arch Neurol. 1990 Mar;47(3):349–355. doi: 10.1001/archneur.1990.00530030131029. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S., Squire L. R., Amaral D. G. Lesions of the amygdala that spare adjacent cortical regions do not impair memory or exacerbate the impairment following lesions of the hippocampal formation. J Neurosci. 1989 Jun;9(6):1922–1936. doi: 10.1523/JNEUROSCI.09-06-01922.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Cramon D. Y., Hebel N., Schuri U. A contribution to the anatomical basis of thalamic amnesia. Brain. 1985 Dec;108(Pt 4):993–1008. doi: 10.1093/brain/108.4.993. [DOI] [PubMed] [Google Scholar]