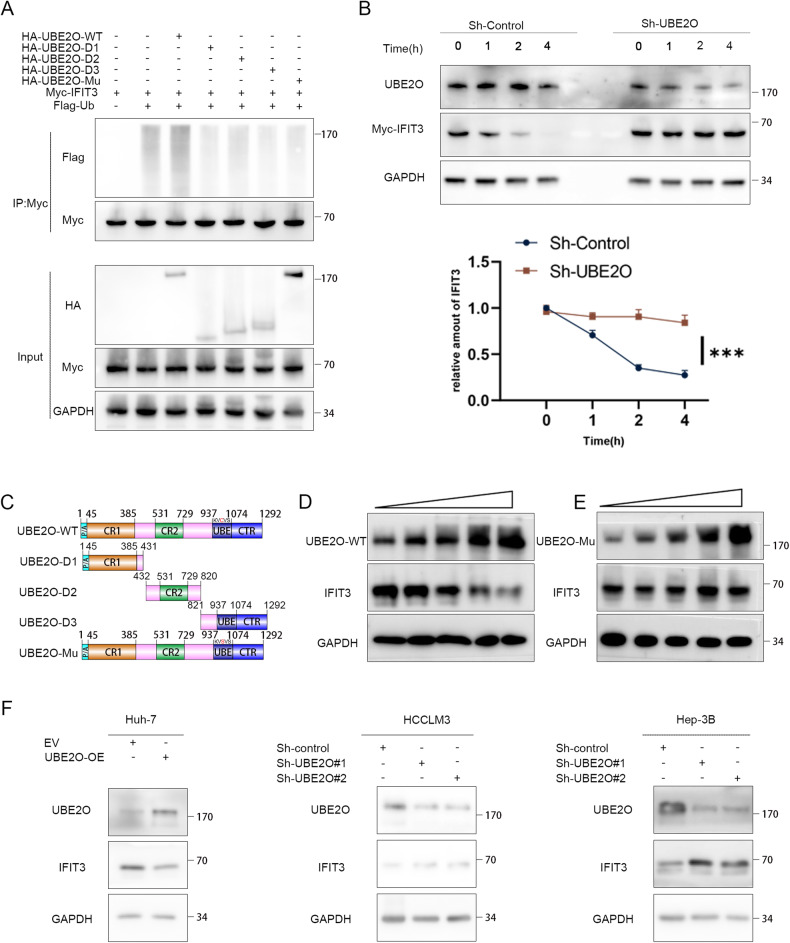
Fig. 4. IFIT3 protein stability is regulated by UBE2O.
A 293 T cells were transfected with the designated plasmids and subsequently exposed to MG132 (10 μM) for a duration of 6 h. The lysed cells were then subjected to coimmunoprecipitation using anti-Myc antibodies, followed by Western blot analysis utilizing anti-Flag antibodies (n = 3). B Stably transfected cells with UBE2O knockdown were established and validated. The cells were treated with cycloheximide (80 μg/mL), and protein was harvested at designated time points for Western blot analysis. Relative band densities were quantified by ImageJ. The data are expressed as the mean ± SD of three independent experiments. Ordinary one-way ANOVA with multiple comparisons testing was used for statistical analysis. *p < 0.05. C Schematic presentation of wild-type UBE2O and the UBE2O mutants. D Gradient overexpression of UBE2O-WT (0.5 µg, 1.5 µg, 2.5 µg, 3.5 µg, and 4.5 µg) was performed, and after 48 h of incubation, the cells were lysed to obtain ~400 ml of total cellular protein per dish for subsequent Western blot analysis of IFIT3 protein expression (n = 3). E Similarly, the amount of the UBE2O-mutant (M3) plasmid transfected was incrementally increased (0.5 µg, 1.5 µg, 2.5 µg, 3.5 µg, 4.5 µg), and the corresponding IFIT3 protein expression levels were measured 48 h posttransfection (n = 3). F In HCCLM3 and Hep-3B cells, knockdown of UBE2O resulted in an increase in the IFIT3 protein level, but overexpression of UBE2O led to a decrease in the IFIT3 protein level in Huh-7 cells (n = 3).