Highlights
-
•
The methods of 21 triazole pesticide residues in animal-origin foods were established.
-
•
A combined modified QuEChERS and LC-MS/MS method was optimized.
-
•
This method is simple, sensitive, rapid, practical and accurate.
-
•
No triazole pesticide residues have been found in animal-origin foods.
Keywords: Triazole fungicide, QuEChERS, UPLC-MS/MS, Animal-origin foods
Abstract
A method for the simultaneous determination of 21 triazole fungicides in animal-origin foods was established by using UPLC–MS/MS. The dilution solvent, extraction solvent, and QuEChERS purification adsorbent composition, were optimized. The response value of the target compound was the highest and the chromatographic peak shape was optimal under the following conditions: water-acetonitrile as the mobile phase, acetonitrile to extract the target compound, C18 (100 mg) as the adsorbent, and water-acetonitrile as the diluent. Our method was validated under electrospray ionization (ESI) + conditions with six animal-origin foods. The 21 triazole fungicides showed good linear relationships (0.1–20 μg∙L−1, R2 > 0.99). The limits of detection and quantitation ranged from 0.1 to 0.3 μg∙kg−1 and 0.3 to 0.9 μg∙kg−1, respectively. The average recoveries ranged from 72.0% to 114.8% with RSDs < 9.9%. Therefore, our method was suitable for the determination of pesticide residues in commercially available animal-origin samples.
1. Introduction
Triazole fungicides are a class of highly effective, low-toxicity, and broad-spectrum endogenous fungicides that are widely applied in the production of vegetables, fruits, and other crops for the prevention and control of various fungal diseases (Han et al., 2014, Zhang et al., 2016). Some of them, such as triadimefon (TDM) and hexaconazole (HEX), are also used as plant growth regulators (Gomathinayagam, Jaleel, Lakshmanan, & Panneerselvam, 2007). However, excessive or improper fertilization in crop planting, processing, and storage has resulted in the presence of residues of these substances in vegetables, fruits, crops, soils, and water bodies, which affect food quality safety and cause environmental pollution. Residual fungicides and their metabolites can be transmitted and enriched through the food chain, accumulating in animal tissues (Anagnostopoulos, Liapis, Haroutounian, & Miliadis, 2013). Since many triazoles may inhibit enzymes involved in the biosynthesis of steroid hormones, which can cause endocrine disorders in humans and wildlife, livestock and poultry products also have safety risks and harmful to consumer health (Ye et al., 2018). Among triazole fungicides, olefinazole, hexazolium, pentazolium, propiconazole, and fluorocycloazole have been listed as possible carcinogens by some international organizations. Japan's “positive list system” (Sun, Gao, & Lian, 2019) and the Codex Alimentarius Commission (CAC) (Li et al., 2011) have set maximum residue limits (MRLs), ranging from 0.01 mg∙kg−1 to 2 mg∙kg−1, for some triazole fungicides in livestock and poultry products.
At present, research on the determination of residual triazole fungicides has mainly focused on water, soil, honey, vegetables, and fruits (Li et al., 2011, Li et al., 2012a, Li et al., 2012b, Mogaddam et al., 2014, Sun et al., 2019, Zhang et al., 2014), however, there are few reports on the determination of triazole fungicides residues in samples of animal-origin foods. Existing sample pretreatment methods include solid phase extraction (SPE) (Li et al., 2019, Miao et al., 2016, Sun et al., 2019), dispersive liquid microextraction (DLLME) (Nie et al., 2016, Wang et al., 2018, Xu et al., 2015), and the quick, easy, cheap, effective, rugged, and safe (QuEChERS) method (Li et al., 2011). The QuEChERS method has been the most widely used method for the determination of pesticide residues in recent years due to its advantages of simplicity, rapidity and use of fewer organic solvents. However, due to the high matrix protein and fat contents of animal products, the QuEChERS method cannot be directly used for pretreatment, so matrix protein and fat should be removed by extraction.
Methods for the detection of triazole fungicide residues have included gas chromatography (GC) (Hildmann et al., 2015, Li et al., 2019), liquid chromatography (LC) (Li et al., 2012a, Li et al., 2012b), gas chromatography–mass spectrometry (GC–MS) (Nie et al., 2016, Wang et al., 2018, Wei et al., 2016), and high-performance liquid chromatography–tandem mass spectrometry (HPLC–MS/MS) (Blondel et al., 2018, Satapute et al., 2019, Zhang et al., 2012). The residue analysis methods for animal food usually adopt more complex SPE column purification or the QuEChERS method combined with other techniques. Camara et al. used GC–MS to analyze 160 pesticide residues in edible snails after purifying samples using a modified QuEChERS method (Camara, Fuster, & Oliva, 2020). Du et al. used acetonitrile extraction and combined purification with gel permeation chromatography and a Carb-NH2 extraction column to establish a GC–MS detection method for 167 pesticide and veterinary drug residues in animal foods such as meat and aquatic products (Du, Lu, Zhu, Miao, & Wu, 2013). To the best of our knowledge, there are almost no analytical studies on the simultaneous determination of triazole fungicides in several different foods of animal origin.
This study focuses on animal-origin food protein, in which the fat content is higher in the matrix, using the improved QuEChERS method, with high purification efficiency and simple operation, for the removal of fat and protein, combined with ultra-performance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS), with high sensitivity and good repeatability. This method can be used for the rapid and accurate determination of triazole fungicides in livestock and poultry products and provide technical support for residue monitoring and quality control of animal-origin food.
2. Materials and methods
2.1. Reagents
Deionized water (>18.2 MΩ) was obtained using a Milli-Q water purification system (Millipore Corp., USA). Formic acid, ethyl acetate, and acetonitrile of LC–MS-grade were purchased from Merck Co. (Darmstadt, Germany). Primary/secondary amine (PSA, 40–63 mm, 6 nm), octadecylsilane (C18), and graphitized carbon black (GCB) were obtained from CNW Technologies GmbH (Düsseldorf, Germany). NaCl was purchased from Kemiou Co. Ltd. (Tianjin, China).
Standards of penconazol (CAS: 66246-88-6), myclobutanil (CAS: 88671-89-0), cyproconazol (CAS: 113096-99-4), uniconazole (CAS: 83657-17-4), flusilazole (CAS: 85509-19-9), triadimefon (CAS: 43121-43-3), triadimenol (CAS: 55219-65-3), flutriafol (CAS: 76674-21-0), fluconazole (CAS: 86386-73-4), tebuconazole (CAS: 107534-96-3), hexaconazole (CAS: 79983-71-4), simeconazole (CAS: 149508-90-7), triticonazole (CAS: 131983-72-7), diniconazole (CAS: 83657-24-3), epoxiconazol (CAS: 106325-08-0), fenbuconazole (CAS: 114369-43-6), propiconazole (CAS: 60207-90-1), voriconazole (CAS: 137234-62-9), tetraconazole (CAS: 112281-77-3), bromuconazole (CAS: 116255-48-2), difenoconazole (CAS: 119446-68-3) were acquired from Alta Scientific Co., Ltd. (Tianjin, China), and their purities were higher than 98%.
Stock solutions of 21 triazole fungicides were prepared using 100 µg∙mL−1 acetonitrile and stored at −20 °C for future use. A mixed standard solution of 1.0 µg∙mL−1 was then prepared in acetonitrile and stored at 4 °C for future use.
2.2. Sample
2.2.1. Sample preparation
Egg, chicken, mutton, beef, pork, and pork liver samples were purchased from different farmers' markets in Shihezi City. A random sampling method was followed during sample collection. First, the samples were cut into small pieces. Then, the homogenate was processed by a tissue masher and placed in a sample storage bag at −20 °C for future use.
2.2.2. Sample pretreatment and optimization methods
Sample pretreatment plays an important role in analytical chemistry. In this study, sample pretreatment mainly included three steps. First, the composition of the dilution solvent was optimized. Water, acetonitrile, methanol, water-acetonitrile, water-methanol, 0.1% formic acid (FA) water, 0.1% FA water-acetonitrile, and 0.1% FA water-methanol were selected as optional solvents. By comparing the effects of the six alternative solvents on the signal response strength and chromatographic peak shape of the triazole fungicides, the optimal dilution solvent was selected. Second, we optimized the composition of the extraction solvent. By comparing the extraction efficiency of acetonitrile and ethyl acetate, the most suitable extraction solvent was selected. Finally, we optimized the QuEChERS purification process. To achieve this, we analyzed the recovery ability of three common adsorbents, C18, PSA, and GCB, for 21 triazole fungicides in 6 samples at two doses (50 mg and 100 mg) and screened out the best sorbent type and dosage.
First, a 2.00 g sample was precisely weighed and added into a centrifuge tube with 10 mL acetonitrile. After homogenization for 1 min at 10,000 r∙min−1, 1 g anhydrous magnesium sulfate was added, and the mixture was vortexed for 1 min. The supernatant was obtained after 5 min of centrifugation at 8000 r∙min−1. Then, 100 mg of C18 adsorbent and 2 mL of supernatant were added into a centrifuge tube. After vortexing and stirring, the mixture was centrifuged at 10,000 r∙min−1 for 3 min. The supernatant was then collected and 1 mL was obtained, dried under nitrogen gas at 40 °C, and dissolved in 1 mL water–acetonitrile (V:V = 9:1). After passing through an organic-phase needle filter, the sample was used for instrumental analysis.
2.3. Chromatographic conditions
A BEH-C18 column (100 mm × 2.1 mm, 1.7 µm, Waters) with a column temperature of 35 °C, an injection volume of 5 μL and a flow rate of 0.3 mL∙min−1 was used. Mobile phase A was water, and mobile phase B was acetonitrile. The gradient elution procedure was as follows: 0 ∼ 0.5 min, 95% A; 0.5 ∼ 3 min, 65% A; 3 ∼ 10 min, 40% A; 10 ∼ 13 min, 10% A; 13.1 min, 95% A. The running time was 20 min.
2.4. Mass spectrometry conditions
The ion source was an electrospray ionization source (ESI+) in multiple reaction monitoring (MRM) ion scanning mode with a capillary voltage of 2.5 kV, a cone hole flow rate of 50 L∙h−1, an ion source temperature of 150 °C, a desolvation gas temperature of 400 °C, and a desolvation gas flow of 800 L∙h−1. The MS experimental parameters for the 21 triazole fungicides are shown in Table S1.
2.5. Method validation
Our method for the simultaneous determination of 21 triazole fungicides in 6 sample matrices was evaluated using the metrics of linearity, precision, accuracy, limit of detection (LOD), limit of quantitation (LOQ), and matrix effects (MEs).
By contrasting the chromatograms of the sample matrix standard and the solvent standard, MEs were assessed. The calculation formula for MEs is as follows:
The precision and accuracy of the method were determined by five repeated assay recoveries of blank sample solution at three intensification levels (1.0, 2.0, 5.0 μg∙kg−1).
The LOD and LOQ of 21 triazole fungicides were determined by continuous gradient dilution of mixed standard solution with blank matrix solution. These two indicators were defined as 3 times and 10 times the signal-to-noise ratio (S/N) of the target analysis, respectively.
Using a blank sample matrix, mixed standard solutions of 21 triazole fungicides were continuously diluted to different concentrations (0.1, 0.2, 0.5, 1.0, 2.0, 5.0, 10.0, 20.0, 50.0, 100.0 μg∙L−1) to evaluate their linearity. By drawing the concentration-peak area curves of 21 triazole fungicides, the linear equation of the matrix matching calibration curve was obtained, and the determination coefficient (R2) was calculated.
3. Results and discussion
3.1. Optimization of the mobile phase
To achieve the effective separation of target analytes, improve detection sensitivity and reduce MEs, we used a C18 column as the separation column to compare the separation performance of 21 triazole fungicides in different mobile phase systems. In this work, we compared the effects of fourteen mobile phases, comprising water, acetonitrile, methanol, ammonium acetate, ammonium formate, and formic acid, on the response and separation effect of the target fungicides. As shown in Fig. 1, all target analytes exhibited higher response values and sharper peak shapes when the acetonitrile system was used as the mobile phase than when the methanol system was used as the mobile phase. This is mainly because the elution ability of methanol is weaker than that of acetonitrile. On the other hand, low-wavelength UV absorption by methanol could decrease the sensitivity of the analytical approach. In addition, in the study of different additives (formic acid, ammonium acetate, and ammonium formate), we found that the target analytes exhibited stronger response signals and sharper peaks without the addition of additives. This is similar to the results reported in earlier studies in which a chromatographic method was used for fungicide determination; these studies showed that higher abundances of target compounds could be detected without the use of mobile phase additives such as ammonium acetate and formic acid (Ma et al., 2017). The reason for this phenomenon may be that some salt is extracted after the mixing of the mobile phase, and the background absorption of salt leads to baseline drift. The baseline substantially drops when the gradient changes at a low wavelength, especially when ammonium acetate is added to the mobile phase, which has a significant impact on the precise measurement of compounds with low contents. As a result, acetonitrile–water was chosen to be the mobile phase in the subsequent experiments.
Fig. 1.
Effect of 12 mobile phases on response of 21 triazole fungicides.
3.2. Optimization of dilution solvent
For chromatographic analysis, the wrong choice of dilution solvent may result in poor peak shape, peak splitting, and unreliable retention times (Otero, Alfonso, Rodriguez, Vieytes, & Botana, 2012). In this study, we chose water, acetonitrile, methanol, formic acid, and their combined systems as dilution solvents for optimization based on the chemical properties of the mobile phase system and triazole fungicides. By comparing the effects of different dilution solvents on the signal response strength and chromatographic peak shape of target compounds, the optimal solvent composition was determined. As shown in Fig. S1, the maximum response value of the target compounds was obtained when a dilute solution of the acetonitrile system was used (acetonitrile > water-acetonitrile > formic-water-acetonitrile). However, when using pure acetonitrile as the dilution solvent, we found that the chromatographic peaks of some of the target compounds were less symmetrical and exhibited a tailing effect. This phenomenon may be due to the poor match between pure acetonitrile and the mobile phase solution. A previous study also showed that adding water to a diluted solution significantly improved the chromatographic peak shape (Xing, Zou, Luo, & Wang, 2020). The peaks of the target compounds were symmetrical, and the signal strength was better when water-acetonitrile and formic-water-acetonitrile were used as diluted solutions. Therefore, in the following tests, the water-acetonitrile solution was chosen as the dilution solvent.
3.3. Optimization of the extraction solvent
The 21 target compounds showed significant polarity differences. Acetonitrile and ethyl acetate are widely used to extract compounds in pesticide residue analysis because of their strong polarity and penetration ability (Hildmann et al., 2015, Nie et al., 2016). In this work, we compared the extraction efficiency of acetonitrile and ethyl acetate for 21 triazole fungicides in 6 sample substrates. The effects of different extraction solvents were determined by using the recovery method. The mixed standard solution of 21 target compounds was added to 6 blank sample substrates at a spiked level of 5 μg∙kg−1. After mixing and standing for a period of time, 20 mL of extraction solution was added. Fig. 2 displays the effectiveness of the two extraction solutions. The average recoveries of acetonitrile and ethyl acetate were 94.3% and 94.8%, respectively. However, the recovery rate of epoxiconazol in eggs, mutton, pork and pig liver was as low as 46.8%-61.2% when ethyl acetate was used as the extraction solvent. The recovery rate of flutriafol in egg, beef, mutton and chicken substrates was 125.7%-132.3%. For acetonitrile, in the six sample substrates, the recoveries of the 21 triazole fungicides varied between 70.9% and 118.7%, and all recoveries were satisfactory. This may be because ethyl acetate is partially miscible with water and cannot completely separate the strongly polar triazole fungicides from the aqueous substrate. Moreover, the coextract of ethyl acetate is relatively large, resulting in a large measurement error. However, acetonitrile is a strong solvent for triazoles, can effectively permeate animal-origin samples, and has relatively few coextracts. This is in line with the findings of a prior study, in which a technique for the assessment of fungicides in ambient water-based samples utilizing acetonitrile as the extraction solvent was described and demonstrated that acetonitrile had a higher extraction efficiency than traditional extraction solvents (Xu et al., 2015). Therefore, acetonitrile is more suitable for the extraction of triazole fungicides from animal-origin samples. Acetonitrile was chosen as the extraction solvent in the following tests.
Fig. 2.
Effects of two extraction solvents on recoveries of 21 triazole fungicides in six animal-origin foods (a) egg, (b) chicken, (c) beef, (d) mutton, (e) pork liver, (f) pork.
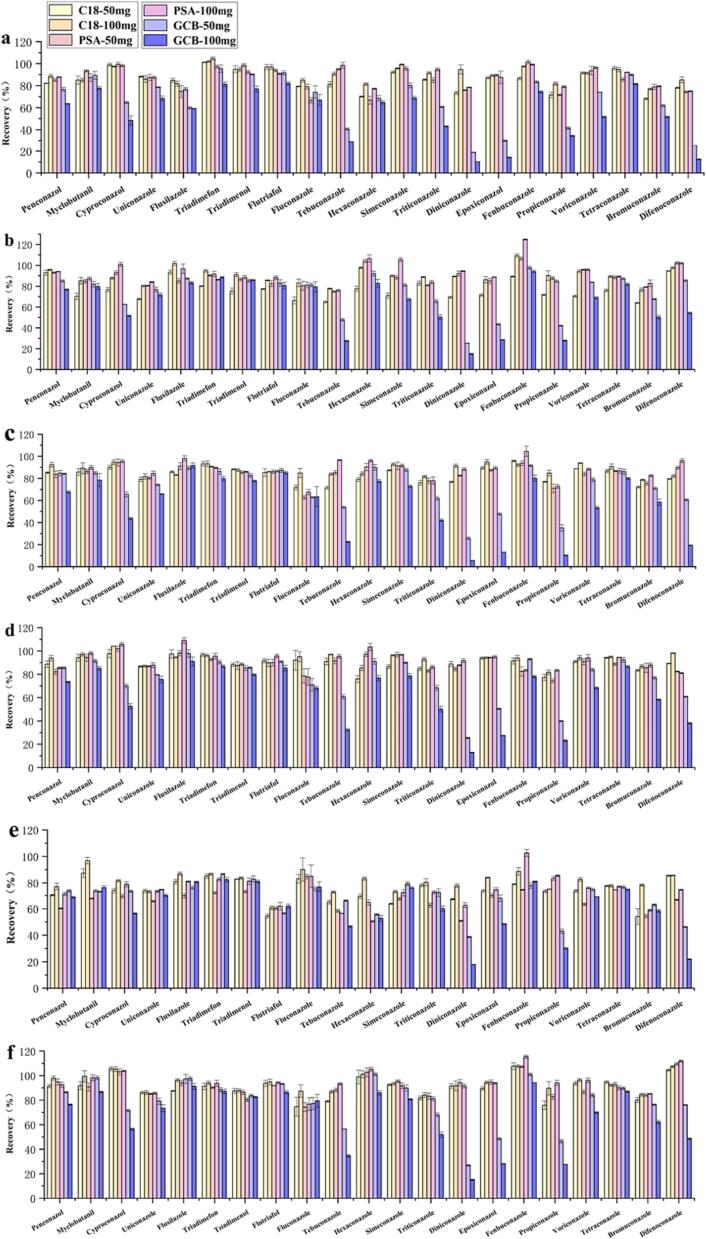
3.4. Optimization of QuEChERS purification
The animal-origin sample matrix is very complex and contains many proteins, lipids, fatty acids, carbohydrates, and other interfering substances. Before doing an analysis with UPLC-MS/MS, the samples must be purified to limit the impact of these chemicals on the results (Wang et al., 2022). QuEChERS purifying technology has been extensively used in the detection of agricultural products (Cao et al., 2019, Zhang et al., 2016). As parameters of the QuEChERS purification process, the selection and dosage of the adsorbent are very important issues. Studies have shown that C18 is beneficial for removing nonpolar interfering substances, such as lipids and sterols (Ma et al., 2017), GCB can strongly adsorb to planar molecules and is beneficial for removing nonpolar substances, such as pigments (Cao et al., 2019), and PSA can remove organic acids, sugars, and other polar interfering substances (Zhang & Xu, 2013). In this study, we examined the ability of GCB, C18, and PSA, three frequently used adsorbents, to recover 21 triazole fungicides at two doses (50 mg and 100 mg) from 6 samples. As shown in Fig. 3, the purification effect of C18 and PSA on 21 triazole fungicide samples was significantly stronger than that of GCB. This may be due to the inability of GCB to remove lipid interfering substances. Moreover, GCB also can strongly adsorb some target analytes. This is in line with the results of Zhang et al., who reported that C18 has stronger scavenging effects on interfering lipids than GCB and is more suitable for the pretreatment of animal samples (Zhang, Wang, Li, & Wang, 2017). In addition, the above studies also reported that GCB has adsorption effects on a variety of pesticides. The recoveries of tebuconazole, texaconazole, diniconazole, and bromuconazole were all lower than 60%, and the average recoveries of 21 fungicides were 67.36% and 73.92%, respectively, when PSA (50 mg and 100 mg) was used for the purification of these fungicides from pig liver. It can be inferred that PSA has a retention effect on the above fungicides, leading to the low recovery rate. The recoveries of flutriafol and bromuconazole were lower than 60%, and the average recoveries of the 21 fungicides were only 73.95% when C18 (50 mg) was used for the purification of these fungicides in pig liver. This indicates that a low dose of C18 has a poor purification effect. However, the recoveries of all target analytes were satisfactory (72.87%-109.62%) when C18 (100 mg) was used to purify the fungicides in all 6 sample matrices, with an average recovery of 89.26%. These results showed that C18 (100 mg) was the best adsorbent for animal-origin samples. Therefore, we chose the C18 (100 mg) adsorbent to purify egg, chicken, beef, lamb, pork, and pig liver samples.
Fig. 3.
Effects of six sorbents on recoveries of 21 triazole fungicides in six animal-origin foods (a) egg, (b) chicken, (c) beef, (d) mutton, (e) pork liver, (f) pork.
3.5. Method validation
Under optimal conditions, the effectiveness of this method was assessed, including in terms of the MEs, LOD, LOQ, linearity, precision, and accuracy.
3.5.1. Matrix effects
MEs are often reported in LC–MS/MS analyses (Shuang, Zhang, Zhong, & Li, 2021). This is mainly because during the sample ionization process, substances from the sample matrix and foreign interfering compounds compete with the target compounds in terms of charge and neutralize the ionization process. MEs can be enhanced or inhibited (Mogaddam et al., 2014). In this work, we determined the MEs of 21 fungicides in 6 sample matrices. The measured ME values were divided into three categories: −20% to 20%, below −20%, and above 20%. When the ME value was between −20% and 20%, its influence was considered to be almost negligible. When the ME value was less than −20% or greater than 20%, matrix inhibition or enhancement effects were considered to be present (Ma et al., 2017). As shown in Fig. S2, most fungicides had ME values in the range of −20% to 20%, with negligible MEs. However, the MEs of fluconazole and tetraconazole were lower than −20%, indicating significant matrix inhibition. This indicates that although we purified the solution, substances still interfered with the analysis of the target compound. To lessen the negative impact of MEs on the analysis, a matrix matching calibration curve was used for analysis in this study.
3.5.2. Selectivity
The selectivity of established detection methods affects the analysis of target compounds. In this work, we compared the chromatograms of 21 triazole fungicides with an added concentration of 5.0 μg∙kg−1 in matrix matching standard solution, blank matrix solution, and egg samples (Fig. 4). The results showed that the residual impurity peaks did not affect the analysis of target compounds after purification by the QuEChERS method. The target components exhibited a large separation degree, which showed that the our proposed method had exceptional selectivity.
Fig. 4.
UPLC-MS/MS in multiple reaction monitoring of 21 triazole fungicides (a) mixed matrix standard solution, (b) blank matrix solution, (c) egg sample at 5.0 μg·kg−1 added concentration.
3.5.3. Trueness and precision
To evaluate the accuracy and precision of the established method for each triazole fungicide, we added each fungicide at concentrations of 1.0, 2.0 and 5.0 μg∙kg−1 to six blank samples (egg, chicken, beef, mutton, pork, pork liver) and determined the recoveries and relative standard deviations (RSDs). As shown in Table 1, the average recovery ranged from 72.0% to 114.8% at the three concentrations. The assay was repeated five times for the standard solution, and the RSDs were lower than 9.9%. The recovery rate and RSDs data were in accordance with the acceptance criteria stipulated in the SANTE/12682/2019 (European Commission, 2017) guidelines (recovery range 70–120%, RSD < 20%), which indicated that our method had good accuracy and repeatability.
Table 1.
Recoveries and RSDs of 21 fungicides in six animal-origin foods substrates at spiked levels.
| Compound | Spiked (μg∙kg−1) |
Egg |
Chicken |
Beef |
Mutton |
Pork |
Pork liver |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | ||
| Penconazol | 1 | 95.2 | 5.1 | 80.2 | 4.2 | 106.0 | 3.0 | 105.5 | 4.5 | 107.5 | 1.8 | 76.5 | 3.4 |
| 2 | 90.8 | 1.3 | 101.2 | 3.0 | 97.0 | 5.8 | 105.9 | 1.2 | 108.5 | 1.2 | 81.5 | 2.8 | |
| 5 | 90.2 | 3.0 | 98.1 | 3.2 | 100.1 | 1.8 | 99.8 | 2.3 | 88.8 | 3.4 | 90.4 | 2.9 | |
| Myclobutanil | 1 | 112.0 | 4.1 | 104.6 | 2.2 | 101.4 | 2.9 | 101.2 | 2.2 | 102.9 | 5.1 | 80.8 | 4.3 |
| 2 | 104.4 | 3.4 | 108.0 | 4.9 | 93.9 | 2.3 | 99.4 | 4.2 | 105.1 | 3.0 | 83.9 | 9.9 | |
| 5 | 104.4 | 4.1 | 96.3 | 2.7 | 98.5 | 0.9 | 95.9 | 2.4 | 87.5 | 3.4 | 96.9 | 1.9 | |
| Cyproconazol | 1 | 114.8 | 4.1 | 94.9 | 4.4 | 100.6 | 1.9 | 99.6 | 3.9 | 108.6 | 3.1 | 87.6 | 2.1 |
| 2 | 106.9 | 1.4 | 113.4 | 2.0 | 83.2 | 2.1 | 93.8 | 2.3 | 108.3 | 1.7 | 89.5 | 3.1 | |
| 5 | 100.2 | 3.8 | 98.6 | 2.9 | 99.4 | 2.3 | 90.5 | 1.8 | 88.5 | 1.6 | 100.3 | 2.7 | |
| Uniconazole | 1 | 107.3 | 4.7 | 104.8 | 2.2 | 103.6 | 2.6 | 102.2 | 2.6 | 111.6 | 2.3 | 89.1 | 4.8 |
| 2 | 102.4 | 2.1 | 97.6 | 1.8 | 86.0 | 1.2 | 99.8 | 2.1 | 100.8 | 2.2 | 85.9 | 3.4 | |
| 5 | 95.6 | 3.6 | 101.6 | 1.3 | 100.6 | 1.7 | 103.0 | 1.7 | 81.3 | 2.4 | 104.9 | 0.9 | |
| Flusilazole | 1 | 105.5 | 3.3 | 100.3 | 7.7 | 100.0 | 1.5 | 103.0 | 2.2 | 108.0 | 2.4 | 78.1 | 3.3 |
| 2 | 105.1 | 2.2 | 107.8 | 1.7 | 85.4 | 2.5 | 102.1 | 2.4 | 106.2 | 1.2 | 83.5 | 2.5 | |
| 5 | 100.5 | 5.2 | 106.5 | 2.6 | 105.8 | 2.3 | 100.1 | 1.1 | 86.3 | 3.1 | 96.3 | 2.2 | |
| Triadimefon | 1 | 90.0 | 5.5 | 107.8 | 3.9 | 88.2 | 6.4 | 84.4 | 6.6 | 84.6 | 2.6 | 80.8 | 3.7 |
| 2 | 89.5 | 8.0 | 102.7 | 3.6 | 87.4 | 3.9 | 107.9 | 5.5 | 100.6 | 6.1 | 84.5 | 2.5 | |
| 5 | 91.8 | 2.3 | 99.4 | 3.3 | 94.6 | 4.2 | 100.2 | 2.0 | 89.2 | 3.1 | 98.9 | 4.0 | |
| Triadimenol | 1 | 86.5 | 2.8 | 107.8 | 2.8 | 90.0 | 7.2 | 86.7 | 5.7 | 93.5 | 5.5 | 83.4 | 5.7 |
| 2 | 91.2 | 5.6 | 104.8 | 2.0 | 94.0 | 3.1 | 98.9 | 1.7 | 116.0 | 4.6 | 85.1 | 3.3 | |
| 5 | 95.4 | 3.9 | 101.7 | 1.4 | 95.9 | 3.8 | 99.9 | 1.2 | 103.3 | 2.2 | 105.5 | 2.3 | |
| Flutriafol | 1 | 109.0 | 3.7 | 102.4 | 4.3 | 103.5 | 3.6 | 100.7 | 3.0 | 100.9 | 2.8 | 82.6 | 2.0 |
| 2 | 107.2 | 2.2 | 100.6 | 1.7 | 96.7 | 3.6 | 98.2 | 2.2 | 106.5 | 2.0 | 85.7 | 2.6 | |
| 5 | 101.2 | 6.3 | 98.6 | 2.5 | 99.5 | 2.6 | 97.7 | 2.6 | 85.8 | 2.2 | 94.9 | 2.2 | |
| Fluconazole | 1 | 94.8 | 3.2 | 84.6 | 6.2 | 80.8 | 4.2 | 84.4 | 3.6 | 115.0 | 5.1 | 75.0 | 3.3 |
| 2 | 90.6 | 6.3 | 89.0 | 4.1 | 90.5 | 3.6 | 85.3 | 3.3 | 99.7 | 6.8 | 86.3 | 3.8 | |
| 5 | 84.1 | 5.0 | 92.7 | 5.1 | 98.5 | 3.4 | 90.0 | 4.5 | 75.7 | 3.1 | 100.0 | 1.9 | |
| Tebuconazole | 1 | 96.4 | 6.5 | 101.7 | 4.4 | 109.2 | 2.4 | 105.1 | 4.0 | 96.4 | 3.9 | 75.7 | 4.1 |
| 2 | 109.7 | 2.7 | 104.8 | 2.3 | 84.3 | 2.3 | 100.6 | 1.6 | 113.0 | 2.6 | 81.9 | 3.1 | |
| 5 | 99.1 | 5.1 | 101.9 | 1.5 | 101.6 | 2.2 | 90.9 | 3.6 | 94.9 | 2.6 | 95.5 | 4.0 | |
| Hexaconazole | 1 | 103.5 | 6.3 | 100.4 | 4.9 | 108.7 | 1.4 | 102.5 | 3.7 | 112.8 | 1.7 | 82.9 | 3.8 |
| 2 | 84.1 | 4.0 | 107.8 | 3.0 | 85.6 | 3.9 | 100.8 | 2.0 | 105.3 | 2.5 | 84.4 | 1.8 | |
| 5 | 92.7 | 6.7 | 99.9 | 1.6 | 101.0 | 0.8 | 94.3 | 2.5 | 82.2 | 1.1 | 91.6 | 3.6 | |
| Simeconazole | 1 | 82.9 | 2.8 | 77.8 | 3.1 | 92.0 | 4.1 | 80.3 | 4.9 | 83.3 | 2.4 | 75.1 | 4.7 |
| 2 | 81.9 | 2.3 | 105.1 | 2.6 | 91.2 | 3.6 | 98.3 | 1.9 | 106.9 | 2.1 | 85.9 | 3.3 | |
| 5 | 92.3 | 3.6 | 101.9 | 3.1 | 101.3 | 1.8 | 102.4 | 1.9 | 86.4 | 3.6 | 103.7 | 1.7 | |
| Triticonazole | 1 | 111.7 | 3.1 | 93.7 | 2.0 | 100.4 | 4.8 | 107.1 | 3.8 | 108.2 | 1.9 | 75.0 | 4.0 |
| 2 | 105.7 | 2.1 | 88.0 | 1.4 | 81.7 | 5.7 | 102.3 | 5.1 | 100.0 | 4.4 | 81.1 | 1.0 | |
| 5 | 93.6 | 2.9 | 95.2 | 2.9 | 93.7 | 4.0 | 100.5 | 3.5 | 81.7 | 2.2 | 90.5 | 4.7 | |
| Diniconazole | 1 | 80.4 | 7.2 | 100.3 | 3.2 | 103.5 | 4.2 | 98.7 | 3.5 | 78.4 | 2.2 | 84.4 | 6.1 |
| 2 | 77.4 | 3.3 | 97.2 | 2.7 | 82.1 | 2.1 | 96.6 | 4.2 | 107.0 | 1.4 | 91.1 | 3.9 | |
| 5 | 76.3 | 4.3 | 101.4 | 3.1 | 98.9 | 2.4 | 100.5 | 1.8 | 91.8 | 2.7 | 100.6 | 3.9 | |
| Epoxiconazol | 1 | 108.3 | 4.0 | 108.0 | 4.7 | 106.1 | 1.6 | 101.7 | 1.5 | 101.4 | 1.7 | 96.9 | 3.5 |
| 2 | 107.2 | 1.8 | 106.8 | 2.0 | 95.2 | 2.6 | 99.6 | 1.1 | 88.0 | 3.0 | 95.4 | 3.6 | |
| 5 | 94.9 | 4.1 | 99.8 | 2.1 | 100.0 | 1.2 | 89.0 | 0.8 | 73.9 | 2.0 | 103.8 | 1.6 | |
| Fenbuconazole | 1 | 92.1 | 3.7 | 89.8 | 3.8 | 99.9 | 2.7 | 90.1 | 3.9 | 99.6 | 3.3 | 99.0 | 5.8 |
| 2 | 86.2 | 2.6 | 102.8 | 5.0 | 101.2 | 2.8 | 82.2 | 2.6 | 100.7 | 5.0 | 94.6 | 3.9 | |
| 5 | 95.8 | 4.1 | 89.5 | 2.9 | 84.1 | 4.1 | 99.0 | 2.6 | 94.6 | 3.5 | 90.9 | 5.0 | |
| Propiconazole | 1 | 87.6 | 2.9 | 90.7 | 2.7 | 92.7 | 5.4 | 93.7 | 3.8 | 85.8 | 4.8 | 76.2 | 1.7 |
| 2 | 91.2 | 5.0 | 99.0 | 2.1 | 75.9 | 3.1 | 92.8 | 3.1 | 106.1 | 3.3 | 86.9 | 3.8 | |
| 5 | 85.1 | 3.1 | 93.0 | 2.4 | 93.4 | 1.4 | 97.8 | 4.6 | 83.6 | 2.3 | 103.4 | 2.9 | |
| Voriconazole | 1 | 112.1 | 2.5 | 101.2 | 3.3 | 111.5 | 2.0 | 105.6 | 5.4 | 97.8 | 4.1 | 93.5 | 3.9 |
| 2 | 104.2 | 2.7 | 103.6 | 2.7 | 99.0 | 3.7 | 101.9 | 3.5 | 100.5 | 2.6 | 94.4 | 4.3 | |
| 5 | 99.2 | 2.5 | 98.8 | 3.2 | 102.0 | 1.8 | 99.6 | 2.4 | 84.6 | 1.6 | 99.8 | 3.1 | |
| Tetraconazole | 1 | 98.7 | 6.1 | 107.7 | 2.5 | 94.1 | 1.6 | 79.6 | 1.3 | 93.8 | 1.7 | 76.2 | 3.1 |
| 2 | 100.0 | 1.3 | 106.6 | 1.1 | 90.0 | 1.1 | 84.4 | 2.8 | 102.1 | 2.3 | 83.6 | 4.3 | |
| 5 | 92.6 | 4.7 | 97.8 | 1.3 | 87.4 | 1.6 | 105.9 | 2.9 | 82.6 | 2.2 | 101.6 | 2.8 | |
| Bromuconazole | 1 | 79.5 | 2.7 | 78.7 | 4.7 | 100.4 | 2.2 | 97.8 | 2.0 | 90.1 | 2.1 | 80.4 | 5.2 |
| 2 | 93.0 | 1.3 | 110.6 | 1.0 | 72.6 | 1.3 | 103.2 | 2.1 | 88.5 | 1.6 | 82.3 | 1.9 | |
| 5 | 96.6 | 3.7 | 105.2 | 2.3 | 103.9 | 1.3 | 99.3 | 1.2 | 90.4 | 1.4 | 100.3 | 1.5 | |
| Difenoconazole | 1 | 93.6 | 2.5 | 72.0 | 1.2 | 87.6 | 1.5 | 81.1 | 2.6 | 91.8 | 2.9 | 75.3 | 2.3 |
| 2 | 105.9 | 2.8 | 76.2 | 3.6 | 81.2 | 1.5 | 76.9 | 0.7 | 83.6 | 1.3 | 80.1 | 3.0 | |
| 5 | 93.1 | 1.8 | 73.5 | 1.6 | 74.7 | 3.0 | 74.1 | 1.6 | 73.8 | 1.4 | 92.9 | 2.3 | |
3.5.4. Linearity, LOD and LOQ
The extraction solution of the blank sample was supplemented with the 21 triazole fungicides with concentration gradients that increased, and the optimal method was utilized for determination. The matrix matching standard curve was plotted against the concentration with the corresponding chromatographic peak areas as horizontal and vertical coordinates. The detection results for the 21 fungicides showed good linear relationships in the concentration range of 0.1–20 μg∙L−1, and the coefficients of determination (R2) were all >0.99.
The minimum peak concentration of the target compound in the sample solution with acceptable accuracy and precision was defined as the LOQ, while the LOD of the established method was defined as the minimum peak concentration that could be detected but not precisely quantified in the sample solution (Ma et al., 2017). With the S/N set at 3 and 10, respectively, the LOD and LOQ were determined in this study. As shown in Table 2, the LOD and LOQ ranged from 0.1 to 0.3 μg∙kg−1 and 0.3 to 0.9 μg∙kg−1, respectively. Compared with reported methods for the detection of triazole fungicides, our method had a lower LOD and LOQ (Zhang et al., 2012, Zhang et al., 2016, Camara et al., 2020).
Table 2.
Performance characteristics of the optimized method.
| Component | Liner range (μg∙L−1) | Linear equation | R2 | LODs (μg∙kg−1) | LOQs (μg∙kg−1) |
|---|---|---|---|---|---|
| Penconazol | 0.1 ∼ 20 | y = 51241.219x + 4657.366 | 0.9997 | 0.10 | 0.30 |
| Myclobutanil | 0.1 ∼ 20 | y = 49946.485x + 10999.694 | 0.9988 | 0.12 | 0.36 |
| Cyproconazol | 0.1 ∼ 20 | y = 231859.640x + 20885.882 | 0.9997 | 0.10 | 0.30 |
| Uniconazole | 0.1 ∼ 20 | y = 75514.179x-2658.992 | 0.9998 | 0.10 | 0.30 |
| Flusilazole | 0.1 ∼ 20 | y = 150500.045x + 33920.103 | 0.9986 | 0.10 | 0.30 |
| Triadimefon | 0.1 ∼ 20 | y = 9186.544x-58.922 | 0.9999 | 0.25 | 0.75 |
| Triadimenol | 0.1 ∼ 20 | y = 10548.999x-1440.487 | 0.9978 | 0.10 | 0.30 |
| Flutriafol | 0.1 ∼ 20 | y = 143432.663x + 6767.538 | 0.9999 | 0.20 | 0.60 |
| Fluconazole | 0.1 ∼ 20 | y = 19019.655x-1637.019 | 0.9979 | 0.30 | 0.90 |
| Tebuconazole | 0.1 ∼ 20 | y = 86025.172–18847.003 | 0.9984 | 0.12 | 0.36 |
| Hexaconazole | 0.1 ∼ 20 | y = 80443.484x + 21374.976 | 0.9958 | 0.25 | 0.75 |
| Simeconazole | 0.1 ∼ 20 | y = 127044.023x + 7315.056 | 0.9998 | 0.30 | 0.90 |
| Triticonazole | 0.1 ∼ 20 | y = 58175.053x + 5183.091 | 0.9998 | 0.10 | 0.30 |
| Diniconazole | 0.1 ∼ 20 | y = 44277.822x + 9612.066 | 0.9989 | 0.30 | 0.90 |
| Epoxiconazol | 0.1 ∼ 20 | y = 217013.767x + 23351.808 | 0.9998 | 0.20 | 0.60 |
| Fenbuconazole | 0.1 ∼ 20 | y = 22225.999x-2054.964 | 0.9979 | 0.30 | 0.90 |
| Propiconazole | 0.1 ∼ 20 | y = 23542.032x-1778.438 | 0.9991 | 0.30 | 0.90 |
| Voriconazole | 0.1 ∼ 20 | y = 111956.515x-11848.615 | 0.9986 | 0.30 | 0.90 |
| Tetraconazole | 0.1 ∼ 20 | y = 179184.865x + 6612.699 | 0.9999 | 0.25 | 0.75 |
| Bromuconazole | 0.1 ∼ 20 | y = 169251.769x + 46909.206 | 0.9976 | 0.30 | 0.90 |
| Difenoconazole | 0.1 ∼ 20 | y = 410692.980x-21715.252 | 0.9996 | 0.25 | 0.75 |
3.6. Application to real samples
The established method was applied to real samples. These samples were purchased at the farmers' market in Shihezi City, China, and sample processing was performed using the method described in Section 2.2. Five samples of each sample matrix (egg, chicken, beef, lamb, pig liver, pork) were used, and a total of 30 samples were analyzed. The concentrations of all 21 target fungicides were below the minimum detection limit (0.1 μg∙kg−1). This method can provide reference and basic data for the establishment of maximum residue limits of fungicides in livestock and poultry products and related risk assessment work.
4. Conclusions
In conclusion, a method for the simultaneous determination of 21 triazole fungicides in animal-origin samples by UPLC–MS/MS was established. Under optimized chromatographic conditions, acetonitrile was used to extract the triazole fungicides from the samples, 100 mg of C18 adsorbent was used to purify them, and then an acetonitrile–water solution was used to dilute them. When applying a matrix matching standard curve for quantitative analysis, this method showed outstanding selectivity, accuracy, and precision. Under ESI + conditions, the detection results for the 21 triazole fungicides showed good linear relationships (0.1–20 μg∙L−1, determination coefficient R2 > 0.99). The LOD and LOQ were 0.1–0.3 μg∙kg−1 and 0.3–0.9 μg∙kg−1, respectively. The recovery rates varied from 72.0% to 114.8% on average, with RSDs < 9.9%. Finally, 30 commercially available animal-origin samples were analyzed, and the concentrations of all triazole fungicides were below the minimum detection limit (0.1 μg∙kg−1). Overall, this newly developed method could effectively and reliably identify pesticide residues in animal-origin samples and ensure the safety of animal-origin foods.
CRediT authorship contribution statement
Lijie Xing: Methodology, Software, Validation, Investigation, Writing – original draft. Yang Liu: Validation, Investigation, Visualization, Writing – original draft. Wenqi Li: Validation, Investigation. Liangjun Zou: Conceptualization, Methodology. Yuan Wang: Conceptualization, Methodology, Validation, Writing – review & editing, Supervision, Funding acquisition. Ruifeng Luo: Conceptualization, Methodology, Validation, Writing – review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by Uygur Autonomous Region agricultural product quality safety risk assessment project, grant number XJFXPG202301.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.100956.
Contributor Information
Yuan Wang, Email: duguyuanyuan@126.com.
Ruifeng Luo, Email: 1785605310@qq.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- Anagnostopoulos C., Liapis K., Haroutounian S.A., Miliadis G.E. Development of an easy multiresidue method for fat-soluble pesticides in animal products using gas chromatography-tandem mass spectrometry. Food Analytical Methods. 2013;7(1):205–216. doi: 10.1007/s12161-013-9620-x. [DOI] [Google Scholar]
- Blondel A., Krings B., Ducat N., Pigeon O. Validation of an analytical method for 1,2,4-triazole in soil using liquid chromatography coupled to electrospray tandem mass spectrometry and monitoring of propiconazole degradation in a batch study. Journal of Chromatography. A. 2018;1562:123–127. doi: 10.1016/j.chroma.2018.05.056. [DOI] [PubMed] [Google Scholar]
- Camara M.A., Fuster A., Oliva J. Determination of pesticide residues in edible snails with QuEChERS coupled to GC-MS/MS. Food Additives and Contaminants Part a-Chemistry Analysis Control Exposure & Risk Assessment. 2020;37(11):1881–1887. doi: 10.1080/19440049.2020.1809720. [DOI] [PubMed] [Google Scholar]
- Cao J., Zheng Y., Kaium A., Liu X., Xu J., Dong F.…Zheng Y. A comparative study of biochar, multiwalled carbon nanotubes and graphitized carbon black as QuEChERS absorbents for the rapid determination of six triazole fungicides by UPLC-MS/MS. International Journal of Environmental Analytical Chemistry. 2019;99(3):209–223. doi: 10.1080/03067319.2019.1586892. [DOI] [Google Scholar]
- Du J., Lü B., Zhu P., Miao H., Wu Y. Determination of 30 organochlorine pesticides in animal-originated food products using combined purification by gel permeation chromatography and solid-phase extraction coupled with gas chromatography-mass spectrometry. Chinese Journal of Chromatography. 2013;31(8):739. doi: 10.3724/sp.j.1123.2013.01036. [DOI] [PubMed] [Google Scholar]
- Gomathinayagam M., Jaleel C.A., Lakshmanan G.M.A., Panneerselvam R. Changes in carbohydrate metabolism by triazole growth regulators in cassava (Manihot esculenta Crantz); effects on tuber production and quality. Comptes Rendus Biologies. 2007;330(9):644–655. doi: 10.1016/j.crvi.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Han W., Zhong C., Liang L., Sun Y., Guan Y., Wang L.…Li J. Electrochemical degradation of triazole fungicides in aqueous solution using TiO2-NTs/SnO2-Sb/PbO2 anode: Experimental and DFT studies. Electrochimica Acta. 2014;130:179–186. doi: 10.1016/j.electacta.2014.02.119. [DOI] [Google Scholar]
- Hildmann F., Gottert C., Frenzel T., Kempe G., Speer K. Pesticide residues in chicken eggs - A sample preparation methodology for analysis by gas and liquid chromatography/tandem mass spectrometry. Journal of Chromatography. A. 2015;1403:1–20. doi: 10.1016/j.chroma.2015.05.024. [DOI] [PubMed] [Google Scholar]
- Li D., He M., Chen B., Hu B. Magnetic porous organic polymers for magnetic solid-phase extraction of triazole fungicides in vegetables prior to their determination by gas chromatography-flame ionization detection. Journal of Chromatography A. 2019;1601:1–8. doi: 10.1016/j.chroma.2019.04.062. [DOI] [PubMed] [Google Scholar]
- Li J., Dong F., Xu J., Liu X., Li Y., Shan W., Zheng Y. Enantioselective determination of triazole fungicide simeconazole in vegetables, fruits, and cereals using modified QuEChERS (quick, easy, cheap, effective, rugged and safe) coupled to gas chromatography/tandem mass spectrometry. Analytica Chimica Acta. 2011;702(1):127–135. doi: 10.1016/j.aca.2011.06.034. [DOI] [PubMed] [Google Scholar]
- Li Y., Dong F., Liu X., Xu J., Li J., Kong Z.…Zheng Y. Simultaneous enantioselective determination of triazole fungicides in soil and water by chiral liquid chromatography/tandem mass spectrometry. Journal of Chromatography A. 2012;1224:51–60. doi: 10.1016/j.chroma.2011.12.044. [DOI] [PubMed] [Google Scholar]
- Li Y., Dong F., Liu X., Xu J., Li J., Kong Z.…Zheng Y. Enantioselective determination of triazole fungicide tebuconazole in vegetables, fruits, soil and water by chiral liquid chromatography/tandem mass spectrometry. Journal of Separation Science. 2012;35(2):206–215. doi: 10.1002/jssc.201100674. [DOI] [PubMed] [Google Scholar]
- Ma S., Yuan X., Zhao P., Sun H., Ye X., Liang N., Zhao L. Trace determination of five triazole fungicide residues in traditional Chinese medicine samples by dispersive solid-phase extraction combined with ultrasound-assisted dispersive liquid-liquid microextraction and UHPLC-MS/MS. Journal of Separation Science. 2017;40(16):3257–3266. doi: 10.1002/jssc.201700250. [DOI] [PubMed] [Google Scholar]
- Miao Q., Wang J., Nie J., Wu H., Liu Y., Li Z., Qian M. Magnetic dispersive solid-phase extraction based on a novel adsorbent for the detection of triazole pesticide residues in honey by HPLC-MS/MS. Analytical Methods. 2016;8(26):5296–5303. doi: 10.1039/c6ay00376a. [DOI] [Google Scholar]
- Mogaddam M.R., Farajzadeh M.A., Ghorbanpour H. Development of a new microextraction method based on elevated temperature dispersive liquid-liquid microextraction for determination of triazole pesticides residues in honey by gas chromatography-nitrogen phosphorus detection. Journal of Chromatography A. 2014;1347:8–16. doi: 10.1016/j.chroma.2014.04.067. [DOI] [PubMed] [Google Scholar]
- Nie J., Chen F., Song Z., Sun C., Li Z., Liu W., Lee M. Large volume of water samples introduced in dispersive liquid-liquid microextraction for the determination of 15 triazole fungicides by gas chromatography-tandem mass spectrometry. Analytical and Bioanalytical Chemistry. 2016;408(26):7461–7471. doi: 10.1007/s00216-016-9835-y. [DOI] [PubMed] [Google Scholar]
- Otero P., Alfonso A., Alfonso C., Rodriguez P., Vieytes M.R., Botana L.M. Response to Comments on “Effect of uncontrolled factors in a validated liquid chromatography-tandem mass spectrometry method question its use as a reference method for marine toxins: major causes for concern”. Analytical Chemistry. 2012;84(1):481–483. doi: 10.1021/ac203054y. [DOI] [PubMed] [Google Scholar]
- European Commission. (2017). SANTE/12682/2019 of 1st January 2020. Guidance document on analytical quality control and method validation procedures for pesticide residues analysis in food and feed. 1-19.
- Satapute P., Kamble M.V., Adhikari S.S., Jogaiah S. Influence of triazole pesticides on tillage soil microbial populations and metabolic changes. The Science of the Total Environment. 2019;651(Pt 2):2334–2344. doi: 10.1016/j.scitotenv.2018.10.099. [DOI] [PubMed] [Google Scholar]
- Shuang Y., Zhang T., Zhong H., Li L. Simultaneous enantiomeric determination of multiple triazole fungicides in fruits and vegetables by chiral liquid chromatography/tandem mass spectrometry on a bridged bis(beta-cyclodextrin)-bonded chiral stationary phase. Food Chemistry. 2021;345 doi: 10.1016/j.foodchem.2020.128842. [DOI] [PubMed] [Google Scholar]
- Sun P., Gao Y., Lian Y. Determination of triazole fungicides in environmental water by magnetic solid-phase extraction coupled with UHPLC-MS/MS. Journal of the Iranian Chemical Society. 2019;16(7):1483–1489. doi: 10.1007/s13738-019-01614-5. [DOI] [Google Scholar]
- Wang P., Zhao Y., Wang X., Yu G.W., Wang J., Li Z.G., Lee M.R. Microwave-assisted-demulsification dispersive liquid-liquid microextraction for the determination of triazole fungicides in water by gas chromatography with mass spectrometry. Journal of Separation Science. 2018;41(24):4498–4505. doi: 10.1002/jssc.201800860. [DOI] [PubMed] [Google Scholar]
- Wang Y., Xing L., Luo R., Li X., Zhang F., Lu S. Modified QuEChERS combined with UPLC-MS/MS to determine eight biogenic amines in Xinjiang smoked horsemeat sausages. Food Science and Technology. 2022;42 doi: 10.1590/fst.93521. [DOI] [Google Scholar]
- Wei Q., Song Z., Nie J., Xia H., Chen F., Li Z., Lee M. Tablet-effervescence-assisted dissolved carbon flotation for the extraction of four triazole fungicides in water by gas chromatography with mass spectrometry. Journal of Separation Science. 2016;39(23):4603–4609. doi: 10.1002/jssc.201600619. [DOI] [PubMed] [Google Scholar]
- Xing L., Zou L., Luo R., Wang Y. Determination of five Alternaria toxins in wolfberry using modified QuEChERS and ultra-high performance liquid chromatography-tandem mass spectrometry. Food Chemistry. 2020;311 doi: 10.1016/j.foodchem.2019.125975. [DOI] [PubMed] [Google Scholar]
- Xu X.-Y., Ye J.-Q., Nie J., Li Z.-G., Lee M.-R. A new liquid–liquid microextraction method by ultrasound assisted salting-out for determination of triazole pesticides in water samples coupled by gas chromatography-mass spectrometry. Analytical Methods. 2015;7(3):1194–1199. doi: 10.1039/c4ay02448f. [DOI] [Google Scholar]
- Ye X., Ma S., Zhang L., Zhao P., Hou X., Zhao L., Liang N. Trace enantioselective determination of triazole fungicides in honey by a sensitive and efficient method. Journal of Food Composition and Analysis. 2018;74:62–70. doi: 10.1016/j.jfca.2018.09.005. [DOI] [Google Scholar]
- Zhang H., Qian M., Wang X., Wang X., Xu H., Qi P.…Wang M. Analysis of tebuconazole and tetraconazole enantiomers by chiral HPLC-MS/MS and application to measure enantioselective degradation in strawberries. Food Analytical Methods. 2012;5(6):1342–1348. doi: 10.1007/s12161-012-9375-9. [DOI] [Google Scholar]
- Zhang H., Qian M., Wang X., Wang X., Xu H., Wang Q., Wang M. HPLC-MS/MS enantioseparation of triazole fungicides using polysaccharide-based stationary phases. Journal of Separation Science. 2012;35(7):773–781. doi: 10.1002/jssc.201100889. [DOI] [PubMed] [Google Scholar]
- Zhang H.L., Wang J.H., Li L., Wang Y. Determination of 103 pesticides and their main metabolites in animal origin food by QuEChERS and liquid chromatography-tandem mass spectrometry. Food Analytical Methods. 2017;10(6):1826–1843. doi: 10.1007/s12161-016-0736-7. [DOI] [Google Scholar]
- Zhang Q., Tian M., Wang M., Shi H., Wang M. Simultaneous enantioselective determination of triazole fungicide flutriafol in vegetables, fruits, wheat, soil, and water by reversed-phase high-performance liquid chromatography. Journal of Agricultural and Food Chemistry. 2014;62(13):2809–2815. doi: 10.1021/jf405689n. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Xu H. Determination of triazoles in tea samples using dispersive solid phase extraction combined with dispersive liquid-liquid microextraction followed by liquid chromatography-tandem mass spectrometry. Food Analytical Methods. 2013;7(1):189–196. doi: 10.1007/s12161-013-9617-5. [DOI] [Google Scholar]
- Zhang Y., Zhang Y., Nie J., Jiao B., Zhao Q. Determination of triazole fungicide residues in fruits by QuEChERS combined with ionic liquid-based dispersive liquid-liquid microextraction: Optimization using response surface methodology. Food Analytical Methods. 2016;9(12):3509–3519. doi: 10.1007/s12161-016-0548-9. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.