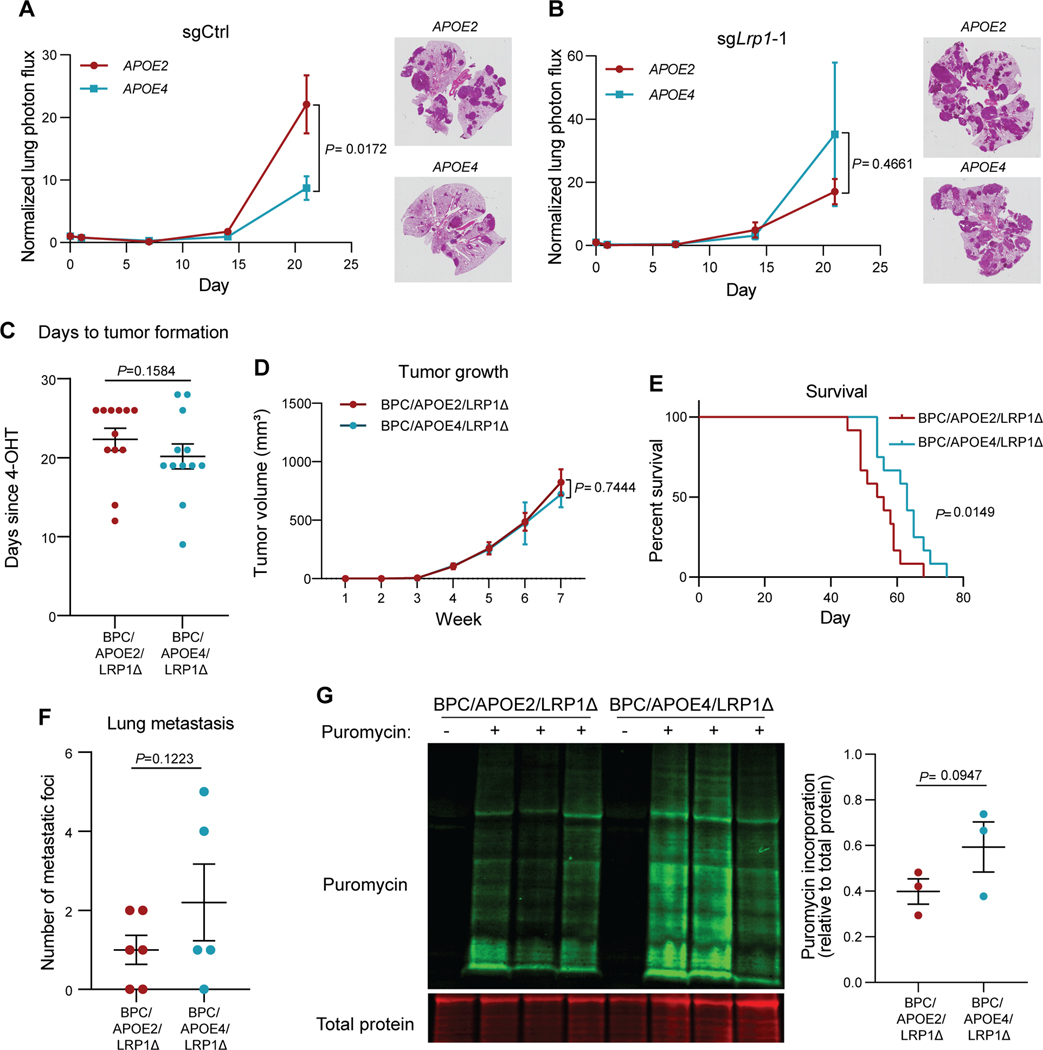
Figure 3. Differential effects of APOE variants on melanoma progression and protein translation are abrogated upon tumoral Lrp1 genetic deletion.
A, B, Quantification of lung metastatic progression via bioluminescence imaging of B16F10-TR-shApoe sgCtrl (A) or sgLrp1-1 (B) cells injected via lateral tail vein into APOE2 and APOE4 mice. Representative images of H&E-stained lungs taken from mice at the day 21 endpoint (n = 9–10 mice per group; representative of two independent experiments; two-way ANOVA). C, Number of days after topical 4-OHT administration until tumors were palpated and visualized in BPC/APOE2/LRP1Δ and BPC/APOE4/LRP1Δ mice (n=12 per group). Unpaired t-test. D, Melanoma tumor growth curves of BPC/APOE2/LRP1Δ (n=12) and BPC/APOE4/LRP1Δ (n=10) mice after topical 4-OHT administration. Two-way ANOVA. E, Kaplan-Meier survival curves of BPC/APOE2/LRP1Δ and BPC/APOE4/LRP1Δ mice after topical 4-OHT administration (n=12 per group). Log-rank test. F, Quantification of lung metastatic foci in BPC/APOE2/LRP1Δ (n=6) and BPC/APOE4/LRP1Δ (n=5) mice after neonatal tumor induction. Unpaired t-test. G, Western blot of puromycin incorporation into BPC/APOE2/LRP1Δ and BPC/APOE4/LRP1Δ tumors 35 days after 4-OHT administration (n=3 per group). Non-puromycin-pulsed mice were included as an antibody control. Unpaired t-test.