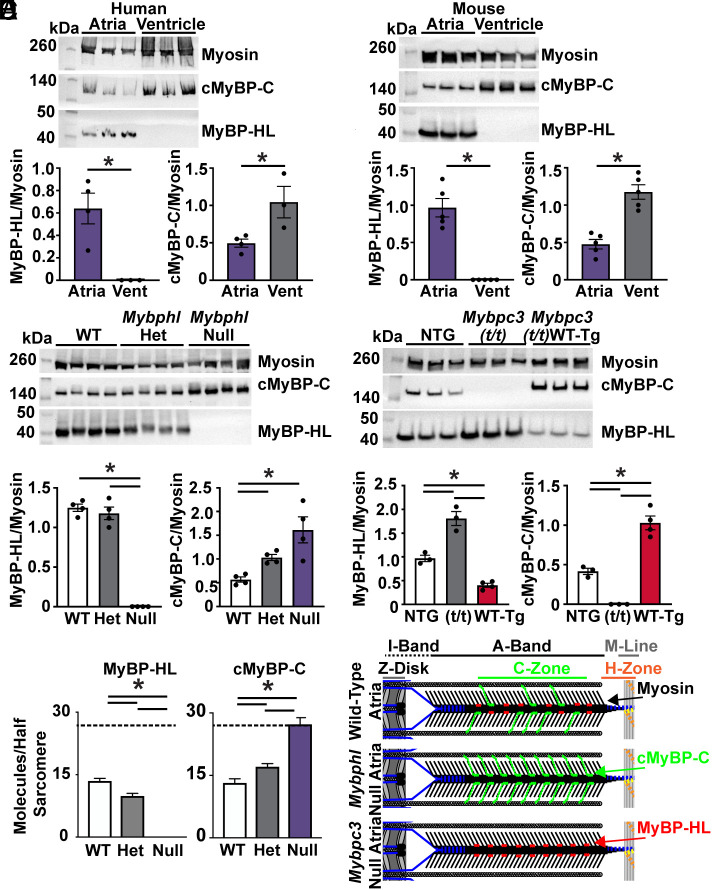
Fig. 2.
MyBP-HL and cMyBP-C have an inverse stoichiometry. (A) Western blot of atrial and ventricular total protein from nonfailing human hearts shows MyBP-HL enriched and cMyBP-C reduced in the atria compared to ventricle; N = 4 atria, 3 ventricles. (B) Western blot of wild-type mouse atrial and ventricular total protein lysate to detect total myosin heavy chain, cMyBP-C, and MyBP-HL shows the atrial enrichment of MyBP-HL, as well a reduction in cMyBP-C levels in the atria; N = 5. (C) Western blot using total atrial protein lysates from WT, heterozygous, and homozygous Mybphl null mice shows an increase in cMyBP-C levels as MyBP-HL is reduced; N = 4. (D) Western blot of total atrial protein lysates from wild-type nontransgenic controls, mice lacking cMyBP-C (Mybpc3 t/t), and mice with a transgenic overexpression of wild-type cMyBP-C (Mybpc3 t/t/WT-Tg) shows that MyBP-HL levels increase or are reduced in an inverse relation to cMyBP-C levels; N = 3 WT, 3 t/t, 4 WT(t/t). (E) Mass spectrometry analysis of total atrial protein from WT, heterozygous, and homozygous Mybphl null mice shows an inverse stoichiometric relationship between MyBP-HL and cMyBP-C, with both protein signals normalized to total myosin content; N = 3. Dashed line at 27 molecules (i.e., full MyBP complement). (F) Schematic of potential inverse stoichiometric binding of cMyBP-C and MyBP-HL in a half sarcomere.