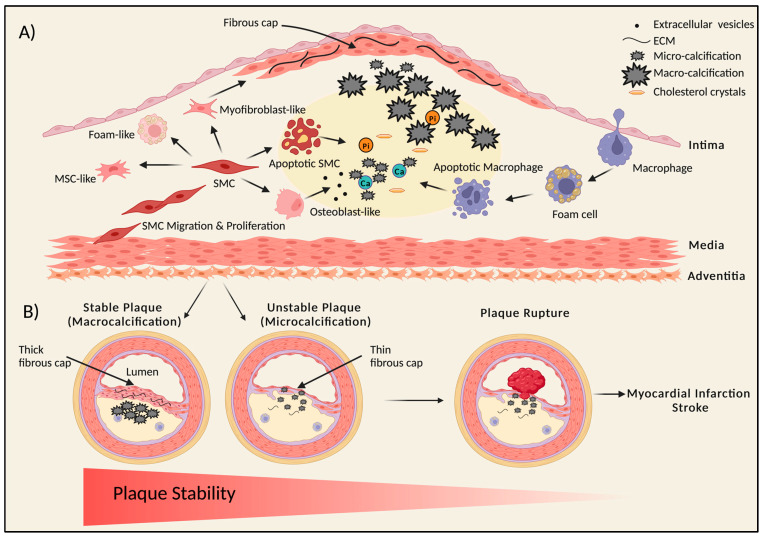
Figure 2.
Schematic representation of plaque initiation, calcification, and progression to atherosclerosis. (A) Initiation and progression of the atherosclerotic plaque and calcification. During atherosclerosis, vascular smooth muscle cells (SMCs) proliferate and migrate to form the fibrous cap stabilizing the plaque. SMCs can give rise to divergent types of cells via transdifferentiation, like osteoblast-like, myofibroblast-like, foam-like, and mesenchymal-stem-like cells within the plaque core. Release of calcifying extracellular vesicles and apoptosis of SMCs leads to the formation of small, calcified deposits called microcalcifications. Monocytes migrate into the intimal thickening via the endothelium, consume the lipids, and mature into foam cells, which can die and also release extracellular vesicles and apoptotic bodies, thus adding to the calcification process. After microcalcification, larger speckles of calcium punctate deposits are formed, causing macrocalcification, which may progress to calcified sheets and plates. These calcified sheets may fracture, leading to nodular calcification, which leads to plaque rupture and thrombosis. Presence of macrocalcifications in the thin fibrous cap can also contribute to plaque rupture. (B) Link between plaque stability and calcification. Stable plaque versus unstable plaque—macrocalcification may lead to stable calcified plaques with a thick collagen-rich extracellular matrix fibrous cap, whereas an unstable plaque has microcalcification with a thin fibrous cap, which is associated with an increased risk of plaque rupture. Microcalcification promotes mechanical stress in the fibrous cap, increasing the propensity to rupture, leading to myocardial infarction or stroke. This figure was created using BioRender.