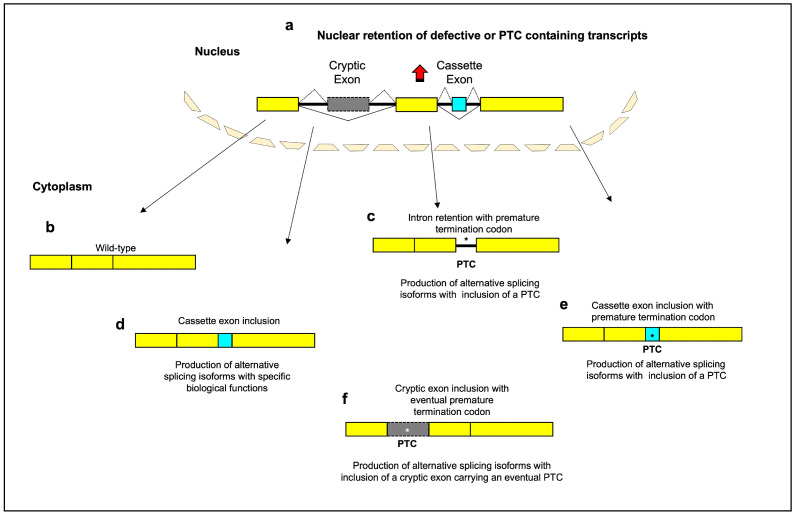
Figure 1.
Schematic diagram of the main aging-related alternative splicing events. Most genes are split between exons and introns. Splicing-defective or premature termination codon (PTC)-containing transcripts are retained within nucleus, with inhibition of translation (a). Normal patterns of splice site selection (indicated with solid black lines) join the constitutive exons together to create “wild type” mRNAs (b). Intron-retaining transcripts can include a PTC and undergo degradation by activation of nonsense-mediated decay (c). The inclusion of a cassette exon into mRNA (indicated with dashed black lines) can lead to a gain of functional domains (d) or to the appearance of a PTC (e). The inclusion of a cryptic exon into mRNA can lead to the introduction of frameshifts or PTC and subsequently to the loss of specific domains or to reduction in gene expression (f). A portion of PTC-containing transcripts is recognized and retained in the nucleus before they have a chance to be exported to the cytoplasm for degradation by the NMD system. PTCs in different reading frames can lead to different levels of nuclear retention. This suggests that the mechanism of nuclear retention is not simply due to the presence of a stop codon, but is dependent on the specific sequence context of the PTC [30,31]. The exact mechanisms by which PTC-containing transcripts are retained in the nucleus are still being investigated. One possible mechanism is alternative splicing—the presence of a PTC can affect the splicing of an mRNA, leading to the inclusion of exons that contain nuclear retention signals that prevent export to the cytoplasm. Another possibility is interaction with specific nuclear proteins or RNA binding proteins that bind to the PTC-containing transcripts and retain them in the nucleus, blocking their export. Changes to the mRNA structure induced by the PTC could also prevent transit to the cytoplasm if the folding prevents export. While further research is needed to elucidate the precise retention mechanisms, the nuclear retention itself may serve a protective purpose. By retaining PTC-containing transcripts in the nucleus, truncated proteins are prevented from being translated in the cytoplasm where they could have detrimental effects on the cell [31,32]. Thus, nuclear retention may act more to protect the cell rather than facilitate mRNA repair pathways like splicing to excise the PTC or induce degradation of unrepairable mRNAs. Yellow box: constitutive exon; gray box: cryptic exon; aqua box: cassette exon; asterisk: premature termination codon.