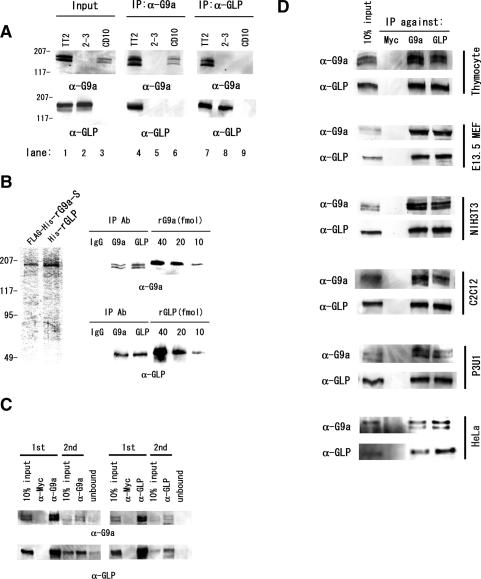
Figure 5.
G9a and GLP form heteromeric complexes. (A) Endogenous G9a and GLP were coprecipitated with specific antibodies against G9a or GLP. (Middle panels) Only the anti-G9a immunocomplexes from wild-type nuclear extracts contain GLP. (Right panels) Similarly, immune complexes from wild-type cells obtained with anti-GLP antibody also contain G9a. (B) Quantitative analyses of anti-G9a and anti-GLP immunocomplexes. (Left) Semipurified recombinant G9a and GLP from virus-infected insect cells were used as protein standards. Amounts of G9a or GLP protein content in immunocomplexes from wild-type nuclear extracts were measured by immunoblotting with precalibrated recombinant G9a or GLP. (Right) Each of the anti-G9a or anti-GLP immunocomplex contains between 10 and 20 fmol of each molecule. (C) G9a/GLP predominantly form heteromeric complexes. Two sequential immunodepletions using anti-G9a (left) or anti-GLP (right) led to a drastic reduction of both proteins from nuclear extracts. (D) G9a and GLP ubiquitously form a heteromeric complex. Anti-G9a and anti-GLP immunocomplexes were prepared from mouse adult thymocytes, E13.5 primary fibroblasts, the NIH3T3 cell line, the C2C12 myocyte cell line, the myeloma cell line P3U1, and the human cell line HeLa and were subjected to Western blot analysis. G9a and GLP formed a heteromeric complex in all murine and human cells tested and this heteromeric complexes were the predominant form.