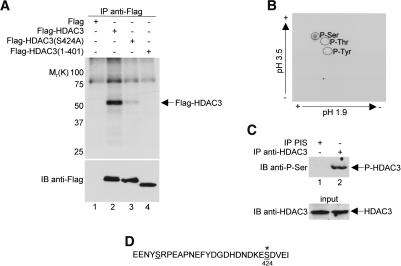
Figure 1.
In vivo phosphorylation of Ser424 on HDAC3. (A) HeLa cells were transfected with Flag control vector or with constructs expressing either wild-type or mutant Flag-HDAC3 and were then labeled with 32P-orthophosphate for 4 h at 37°C. Cell extracts were immunoprecipitated (IP) with anti-Flag antibody under high-stringency conditions, and immunoprecipitates were resolved by SDS-PAGE. (Top panel) Phosphoproteins were visualized by autoradiography. Molecular weight markers positions are indicated on the left. (Bottom panel) Immunoprecipitates were immunoblotted (IB) with anti-Flag antibodies to control for expression efficiency in lanes 2–4. (B) Immunoprecipitated and radiolabeled Flag-HDAC3 was excised from an SDS gel, subjected to partial acid hydrolysis and analyzed by cellulose thin-layer chromatography. (C) HeLa cell extracts were either immunoprecipitated with preimmune sera or anti-HDAC3 antibodies and then immunoblotted with anti-phosphoserine antibodies (top panel) or directly immunoblotted with anti-HDAC3 antibodies (bottom panel). (D) Amino acid sequence of the extreme C-terminal end (residues 401–428) of human HDAC3 (Yang et al. 1997). Putative serine phosphorylation sites, as determined by phosphobase detection (http://www.cbs.dtu.dk/databases/PhosphoBase/predict/predict.html), are underlined. Ser424 is highlighted by an asterisk, indicating its strong likelihood as a protein kinase CK2 phosphorylation site.