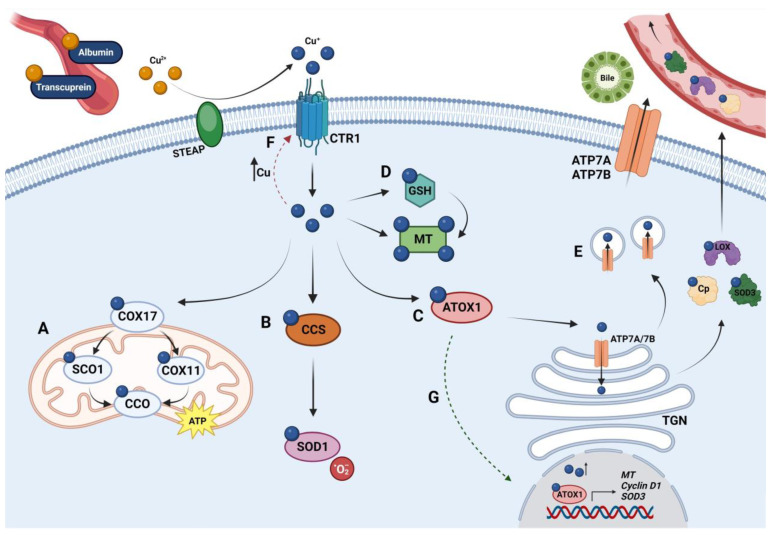
Figure 1.
Schematic diagram of copper metabolism in mammals. After intestinal absorption, Cu travels through the portal vein bound to soluble proteins, such as albumin and transcuprein. On the surface of mammalian cells, metalloreductases such as STEAP 2, 3, and 4 reduce Cu2+ ions to Cu+ so that cells can absorb Cu through CTR1. (A) In the mitochondrial intermembrane space, COX17 is responsible for delivering Cu+ to either SCO1 or COX11 to contribute to the correct assembly of CCO, which utilizes Cu for energy production through oxidative phosphorylation. (B) CCS chaperone transfers Cu+ to SOD1, which is critical in the defense against oxidative stress because it catalyzes the degradation of superoxide radicals. (C) ATOX1 is responsible for providing Cu to the ATPases (ATP7A and ATP7B) that are principally located in the trans-Golgi network (TGN). ATPases pump Cu+ from the cytosol into the lumen of the TGN to promote the synthesis of cuproenzymes, such as Cp, LOX, and SOD3, which are secreted out of the cells to mediate the Cu transport through the circulatory system. (D) Since free Cu ions have the potential to generate reactive oxygen species, excess intracellular Cu+ is sequestered mainly by glutathione (GSH) and metallothioneins (MTs) that uptake Cu for storage. GSH can also deliver Cu to MTs. (E) When the cytoplasmic Cu concentration increases, ATP7A and ATP7B move within endocytic vesicles toward the plasma membrane to transfer excess Cu into the bloodstream. ATP7A is expressed in many tissues except in the liver, where it is replaced by ATP7B. In hepatocytes, ATP7B ensures the movement of Cu through the canalicular membrane for its subsequent elimination through the bile. (F) The concentration of mammalian CTR1 at the plasma membrane is negatively regulated in response to elevated Cu levels (red dotted arrow), with CTR1 being removed from the cell surface. (G) ATOX1 can carry Cu into the cell nucleus and act as a transcription factor for the expression of genes encoding cyclin D1 and SOD3 (green dotted arrow). High concentrations of cellular Cu may also stimulate the transcription of MT genes. Created with BioRender.com (accessed on 11 November 2023).