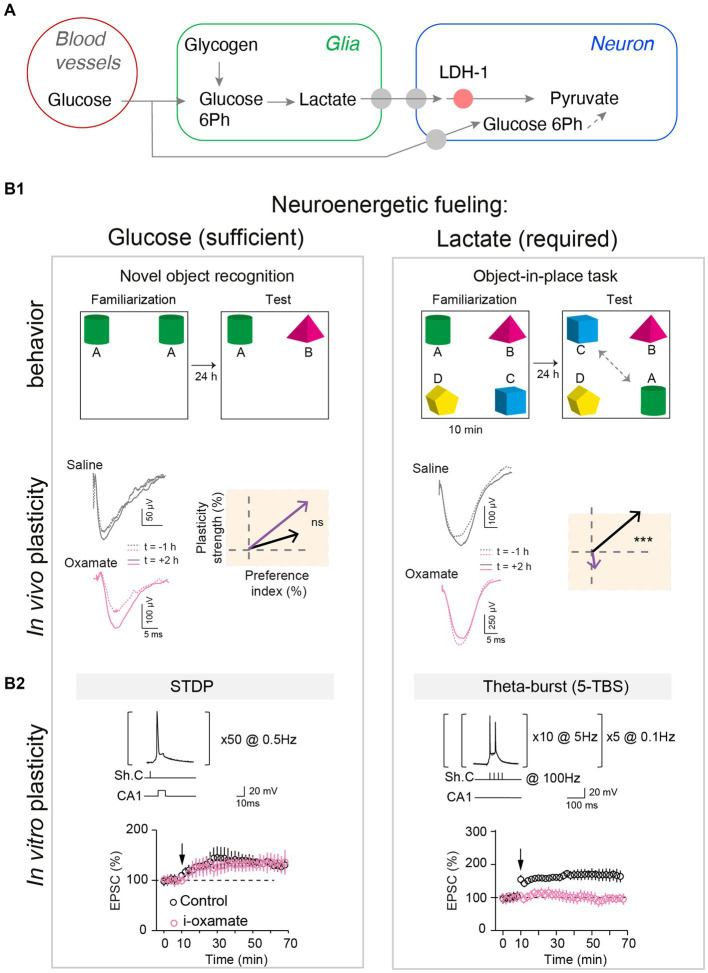
Figure 2.
Glucose and lactate metabolisms are differently engaged in neuronal fueling for plasticity expression and memory. (A) Main steps of the glucose and the glia-derived lactate transports: astrocytic glycogen catalysis into glucose-6-phosphate and then lactate, lactate entry in neurons via monocarboxylate transporters, and lactate conversion into pyruvate by the neuronal lactate dehydrogenase (LDH-1). (B1) Lactate metabolism is necessary for learning cognitive tasks requiring high attentional load as exemplified in the object-in-place task (with four objects) and for expressing the corresponding in vivo hippocampal LTP, but glucose is sufficient for a less demanding task such as a simple novel object recognition (with two objects). Rats were injected bilaterally, via cannulas implanted above hippocampal CA1 layer, with either saline or oxamate (50 mM), an inhibitor of the neuronal LDH preventing the conversion of lactate into pyruvate, before familiarization step. Rats with saline performed equally well in both tasks whereas rats receiving oxamate did not detect novelty in the object-in-place task (illustrated by a low preference index value) and did not express LTP (averaged vectors: y-axis indicates LTP versus LTD expression and x-axis the learning performance evaluated with the preference index). In vivo synaptic plasticity during behavioral task with evoked-field-EPSP recorded before familiarization (baseline) and 2 hours after familiarization to determine synaptic changes, in relation with behavior. (B2) Lactate metabolism is mandatory to fuel the demanding neural computations implicated in NMDA receptor-mediated LTP forms in hippocampus triggered by theta-burst stimulations, while glucose metabolism is sufficient for lighter forms of LTP, based on less and lower-frequency stimulations. The structure of the plasticity induction protocols and the averaged time-course of the synaptic weight after theta-burst stimulation and STDP protocols are illustrated. Oxamate was applied intracellularly (via the patch-clamp pipette) in the sole recorded neuron, and LDH inhibition shows distinct effects on theta-burst stimulation and STDP expression since it prevented theta-burst stimulation-induced LTP but not STDP-induced LTP. In conclusion, scaling of the computational and cognitive loads requires the metabolism of glia-derived lactate to match the neuroenergetic needs of sustained neuronal activity patterns and high cognitive load, and for less demanding plasticity and learning paradigms, glucose suffices as an energy substrate. Adapted from Dembitskaya et al. (2022).