Abstract
Introduction: Hemoptysis is one of the most common symptoms of respiratory system diseases. Common causes include bronchiectasis, tumors, tuberculosis, aspergilloma, and cystic fibrosis. The severity of hemoptysis varies from mild to moderate to massive hemoptysis and can easily lead to hemodynamic instability and death from suffocation or shock. Nevertheless, the most threatening hemoptysis that is presented to the emergency department and requires hospitalization is the massive one. In these cases, today, the most common way to manage hemoptysis is bronchial artery embolization (BAE). Methods: A systematic literature search was conducted in PubMed and Scopus from January 2017 (with the aim of selecting the newest possible reports in the literature) until May 2023 for studies reporting massive hemoptysis. All studies that included technical and clinical success rates of hemoptysis management, as well as rebleeding and mortality rates, were included. A proportional meta-analysis was conducted using a random-effects model. Results: Of the 30 studies included in this systematic review, 26 used bronchial artery embolization as a means of treating hemoptysis, with very high levels of both technical and clinical success (greater than 73.7% and 84.2%, respectively). However, in cases where it was not possible to use bronchial artery embolization, alternative methods were used, such as dual-vessel intervention (80% technical success rate and 66.7% clinical success rate), customized endobronchial silicone blockers (92.3% technical success rate and 92.3% clinical success rate), antifibrinolytic agents (50% clinical success rate), and percutaneous transthoracic embolization (93.1% technical success rate and 88.9% clinical success rate), which all had high success rates apart from antifibrinolytic agents. Of the 2467 patients included in these studies, 341 experienced rebleeding during the follow-up period, while 354 other complications occurred, including chest discomfort, fever, dysphagia, and paresis. A total of 89 patients died after an episode of massive hemoptysis or during the follow-up period. The results of the meta-analysis showed a pooled technical success of bronchial artery embolization equal to 97.22% and a pooled clinical success equal to 92.46%. The pooled recurrence was calculated to be 21.46%, while the mortality was 3.5%. These results confirm the ability of bronchial artery embolization in the treatment of massive hemoptysis but also emphasize the high rate of recurrence following the intervention, as well as the risk of death. Conclusion: In conclusion, massive hemoptysis can be treated with great clinical and technical success using bronchial artery embolization, reducing mortality. Mortality has now been reduced to a small percentage of cases.
Keywords: massive hemoptysis, management, bronchial artery embolization, systematic review, meta-analysis
1. Introduction
Respiratory diseases are among the most common reasons for patients visiting hospitals and emergency rooms. These diseases are most commonly manifested by one or more symptoms: cough, chest pain, shortness of breath, or hemoptysis [1]. Further, the SARS-CoV-2 virus infection that broke out recently and quickly spread in a pandemic appears with these symptoms (most commonly, cough and shortness of breath) [2,3]. Hemoptysis may therefore not be the most common clinical finding in COVID-19 patients [4], but it is an urgent situation that requires observation, finding the cause, and taking steps to prevent a more severe recurrence.
Hemoptysis is defined as the expulsion of blood through the oral tract from a source located in the lower respiratory tract, i.e., below the level of the glottis [5,6]. It is a frequent clinical finding that is usually due to bronchitis, pneumonia, bronchiectasis, tuberculosis, neoplasms (bronchial carcinoma, intrabronchial tumors, sarcomas, and metastases), or even pulmonary embolism [5,6,7,8,9,10]. Other less common causes include coagulopathy, platelet disorders, pulmonary hypertension, aneurysm rupture, trauma (catheter, biopsy, iatrogenic, or blunt trauma), foreign body aspiration, vascular and collagen diseases (such as Goodpasture’s syndrome, Behçet’s disease, systemic lupus erythematosus, granulomatosis with polyangiitis, Henoch–Schonlein purpura, and rheumatoid arthritis), drugs and narcotics (such as anticoagulants, penicillamine, solvents, crack, and cocaine), and even gastric content aspiration or cryptogenic causes [5,6,7,8,9,10,11,12]. Its extent can vary from the presence of blood in the sputum to potentially life-threatening massive hemoptysis [8]. The etiology of hemoptysis affects the choice of appropriate treatment [5,6,9,11].
Massive hemoptysis is defined as blood loss that exceeds 600 mL per twenty-four hours or 150 mL per hour [8]. Massive hemoptysis is related to pathologies that alter the pulmonary vasculature, usually affecting the bronchial arteries, and can be either inflammatory or neoplastic. This emergency can easily and quickly lead to death due to airway obstruction and hypoxemia from asphyxiation, rather than hemodynamic disturbance and shock, which do not occur early [9]. Even a tiny vessel rupture can have significant clinical consequences because of aspiration and the difficulty of stable thrombus formation that prevents vessel healing in thin-walled airways. Due to the severity of the situation, it is necessary to differentiate between the conditions with a similar picture which are characterized as pseudohemoptysis [13]. These can be upper digestive, upper respiratory, or even oral cavity bleeding. Hemoptysis has a bright red color as well as an alkaline frothy sputum, while in other cases of pseudohemoptysis the blood is acidic and darker in color [10,13].
Two pathways are blamed as pathophysiological mechanisms. The first and most common one concerns bleeding from the bronchial arteries because they handle higher pressures [10,14,15,16,17]. This pathway begins when diseases cause hypoxic vasospasm and thereby limit pulmonary circulation. This implies an increase in blood supply with an increase in the rupture of the fragile anastomotic vessels and bleeding into the alveoli and bronchi. Other causative diseases are those that, through the release of angiogenic growth factors, cause neovascularization and the development of collateral circulation. These vessels are also fragile, and so their rupture causes hemoptysis. Hemoptysis, however, can also occur from the vessels of the pulmonary circulation, either iatrogenically during catheterization, during aneurysm rupture, or from other causes [10,14,15,16,17].
According to the guidelines [5,9,10,11,18], the first step is to diagnose massive hemoptysis. The diagnostic algorithm includes a general blood test as well as a coagulation profile. The imaging examination usually does not start with an X-ray because the diagnostic accuracy and detection of bleeding are poor. For this reason, other imaging tests are preferred. These include bronchoscopy, which can detect bleeding in over 90% of cases. Another advantage of this examination is that it does not require moving the patient, while at the same time it offers information about the patient’s anatomy, a fact that facilitates subsequent intervention, increasing its effectiveness [19,20,21]. An alternative method is computed tomography (CT), which has been shown to be even more effective in detecting bleeding and can therefore replace bronchoscopy. Although it is an unusual finding, bronchial artery bleeding can be seen at the time of the scan. This can indicate the site and cause of the bleeding. Native CT can also indicate the site of bleeding, which may manifest as a ground glass infiltration or as pulmonary consolidation, and permit the identification of the site of major architectural distortion in the case of diffuse pathologies [22]. CT angiography permits the identification of the origin of bronchial arteries from the thoracic aorta and facilitates catheterization during interventional angiography. A bronchial artery diameter greater than 3 mm is usually considered pathologic. There is great variability in the number and site of origin of the bronchial arteries, but the most usual patterns are one or two arteries for each lung that originate from the descending thoracic aorta around the level of the tracheal bifurcation. Sometimes, a bronchial artery may arise as a common trunk with an intercostal artery. On the other hand, lung pathology located superficially may result in neovascularization from intercostal artery branches or from internal mammary arteries [5,9,10,16,23,24,25].
Then, according to the guidelines, the hemoptysis is treated [26,27,28]. The treatment of massive hemoptysis has two aspects. The first concerns the emergency arrest of bleeding which does not stop spontaneously and constitutes an emergency. This can be accomplished by using a rigid bronchoscope and adequate aspiration of blood, as well as intubation of the lung and occlusion of the bleeding site, e.g., with a balloon, to ensure a non-bleeding lung. Finally, hemostatic drugs, such as pituitrin, hemocoagulase, and others such as carbazochrome sulfonate sodium and carbazochrome tablets, are applied. Therefore, after the diagnosis is made and emergency treatment is applied, where necessary, follow-up targeted treatment of massive hemoptysis is required. In this phase, the main technique used is bronchial artery embolization (BAE). Alternatives are surgical treatment of the bleeding or etiological treatment [5,9,10,11,18]. The prognosis of massive hemoptysis is indicated by several factors. These include the amount of blood in the sputum, the etiology of hemoptysis (such as cancer), and hemodynamic instability [29,30].
Furthermore, for the treatment of massive hemoptysis, it is important to take certain factors into serious consideration. These include coagulation parameters as well as the taking of anticoagulant drugs. Specifically, the number of platelets determines the safety of both biopsy (safety thresholds of more than 50,000/mm3) and bronchoscopy (safety thresholds of more than 20,000/mm3) [31,32]. Anticoagulation, especially colpidogrel, also increases the risk of bleeding during procedures such as biopsies [33]. From the laboratory findings, urea and BUN are also considered important, as uremia and BUN > 30 mg/dL increase the risk of bleeding during surgery [34].
Since massive hemoptysis is a life-threatening condition, an effective treatment method is essential. Massive hemoptysis, unlike mild hemoptysis, is primarily treated invasively. BAE is considered the method of choice and the first in line. This method can be applied with various techniques. One of the most common is the Seldinger technique. The Seldinger technique usually uses the right femoral artery for access and selective catheterization of the bronchial and intercostal arteries using a 5 French catheter (Cobra, Simmons or DESLER), which is inserted through the diagnostic catheter a few centimeters distally to the vessel origin. This setup provides stability and impedes reflux of embolic material to the thoracic aorta or into radiculomedullary vessels, which, when present, arise as proximal branches [35,36,37]. Catheterization of bronchial arteries and contrast injection will demonstrate enlarged, tortuous vessels with intense angiographic blush in the parenchymal phase of the angiography. This pathologic imaging pattern is enough to justify embolization, given that active contrast extravasation is usually not observed. Each angiographic run should also be thoroughly examined to identify possible branches directed to the spinal canal. Sometimes, radiculomedullary arteries may arise from bronchial arteries as anatomic variations or from a costobronchial trunk. The dominant radiculomedullary artery is the artery of Adamkievitch and provides a major contribution to the anterior spinal artery. This branch presents a characteristic hairpin configuration and projects on the vertebral column along the midline. If angiographic interpretation is difficult, rotational angiography with 3D reconstructions might help. Superselective catheterization of anomalous arteries using 2-F microcatheters is chosen, where possible, for the best results. Several different techniques for access and embolization may be used as appropriate. Also, embolic agents, such as polyvinyl alcohol, coils, gelatin sponges, and Embospheres, are more common [35,36,37]. The embolic materials of choice are particles or microspheres. They should not be smaller than 250 microns, otherwise the probability of their entering dangerous collaterals, leading to non-target embolization, is increased. Coils should be avoided because a new embolization session in case of a relapse will be impossible [17]. It is important to identify and embolize the bronchial artery that supplies the responsible pulmonary lobe, but in cases of diffuse pathologies, embolization of all identified pathologic vessels can be considered, case by case, to prevent future bleeding episodes. The double vascularization of the pulmonary parenchyma usually prevents ischemic complications.
Therefore, knowledge regarding the treatment of massive hemoptysis is necessary to reduce mortality risk. This systematic review aims to evaluate the available treatments for massive hemoptysis according to the latest available studies in the literature, including evaluation of the technical and clinical success of the methods for managing hemoptysis, as well as recurrence, complications, and mortality. The technical success of an operation is defined as successful intervention in massive hemoptysis, i.e., successful bronchial embolization of the bleeding arteries and arrest of the bleeding. Clinical success is defined as the resolution of the clinical findings of massive hemoptysis after the application of the intervention, that is, the cessation of hemoptysis and other clinical findings that the patient may present.
2. Materials and Methods
2.1. Study Protocol and Guidelines
This systematic review was written according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [38]. This systematic review is registered in the Open Science Framework (OSF) with the registration number: DOI osf.io/r2n8w.
2.2. Eligibility Criteria
The eligibility criteria for this systematic review are presented and were defined with the PICO framework. To be included in this systematic review, studies had to meet the following inclusion criteria:
Involve adult patients with massive hemoptysis;
Report on the management of massive hemoptysis;
Provide technical and clinical success rates of the treatment used;
Include data on recurrence and mortality.
Reviews, letters, comments, and case reports were excluded. Only original articles that met the above criteria were included.
2.3. Information Sources, Search Strategy, and Selection Process
A systematic literature search was conducted in two databases (PubMed and Scopus) using the keywords ‘massive hemoptysis’ and ‘management’. The search was limited to a six-year period from January 2017 (with the aim of selecting the newest possible studies in the literature) until May 2023 and articles had to be written in English to be selected. The selection process was carried out by two independent reviewers (D.T. and E. Kar.) using Rayyan [39], who assessed the studies for inclusion in this systematic review. The two reviewers were blinded during both the title and abstract screening and the full-text screening. Any disagreement was resolved by a third reviewer (E. Kot.).
2.4. Data Collection Process and Data Items
Data extraction was carried out by one reviewer (D.T.), and another reviewer (E.Kot.) independently checked the results. The data were extracted in a standardized Excel form. Data extraction was performed without the use of any automation tools. From the studies that were reviewed, the relevant data for extraction were: the main characteristics of each study; the identity of each study (author and year of publication); the total number of participants, along with the mean age and the percentage of men; the type of study; the management of bleeding; and the technical success rate of the method used.
2.5. Quality Assessment
Quality assessment of the included studies was performed using the Newcastle–Ottawa Quality Assessment Scale for Cohort Studies by two independent reviewers (D.T. and E.Kar.). Any disagreement was resolved by a third reviewer (E.Kot.). The Newcastle–Ottawa tool consists of three evaluation domains. These are the selection of the participating patients, comparability, and outcomes. The selection of participants includes: the representativeness of the exposed cohort, the selection of the non-exposed cohort, the ascertainment of exposure, and a demonstration that the outcome of interest was not present at the start of the study. With respect to the first question regarding the representativeness of the exposed cohort, a star is given when the sample is truly or somewhat representative. Regarding the selection of the non-exposed cohort, a star is given if the selection of the non-exposed cohort is drawn from the same community as the exposed cohort. To be given a star for the ascertainment of exposure, a secure record or structured interviews must be presented. Finally, the last star is given if there is a demonstration that the outcome of interest was not present at the start of the study. Regarding the 2nd domain, on comparability, two stars may be given for the comparability of cohorts if the design or analysis controlled for confounders. For the outcomes, the assessment of outcomes and the follow-ups are evaluated. For the first, a star is given if the assessment was independent and blind or a record linkage, while for the follow-up, a star is given if the assessment was sufficient to evaluate the results and if several of the participants were included in it.
2.6. Statistical Analysis
Statistical analysis was conducted with R studio (R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/ (accessed on 8 September 2023)). A proportional meta-analysis was conducted using the metafor [40] and meta packages [41] using a random-effects model. Cases and total numbers of participants were extracted for each outcome from each individual study, and their proportions were calculated during the meta-analysis. Publication bias was determined with visual inspection of funnel plots for each outcome and Egger’s test [42]. Heterogeneity is presented with I2 results, and results between 0 and 40% were considered instances of non-important heterogeneity, those between 40 and 60% instances of moderate heterogeneity, those between 60 and 75% instances of substantial heterogeneity, and those between 75 and 100% instances of considerable heterogeneity [43,44].
3. Results
3.1. Study Selection
A total of 785 studies were found using the keywords ‘treatment’ and ‘massive hemoptysis’, 395 of them in PubMed and 390 in Scopus. Of these, 658 were excluded, as they were either duplicates or letters, case reports, reviews, or comments. Thus, the remaining 127 studies were screened for eligibility, 30 of which met the inclusion criteria and were assessed for eligibility in the full-text screening. These studies therefore analyzed the success rate of the management of massive hemoptysis. The screening process for the studies selected for inclusion in this systematic review is illustrated in a PRISMA 2020 flow chart (Figure 1). All selection stages from the initial stage to the final stage are depicted.
Figure 1.
PRISMA 2020 flow chart. This diagram illustrates the process followed for the collection and selection of studies used in our systematic review.
3.2. Study Characteristics
3.2.1. Major Characteristics of the Included Studies
The 30 studies included 2647 patients who developed massive hemoptysis and were treated accordingly [45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74]. In these studies, treatment was applied either to stop bleeding in cases of massive hemoptysis in which conservative treatment was unsuccessful or to prevent recurrent bleeding. All these studies were retrospective observational cohort studies. The largest sample size was 489 patients, in Ishikawa et al., 2017 [49]. This study was conducted in Japan by a center specializing in hemoptysis. In contrast, the smallest sample size was found in Clements et al., 2022 [72], with only three patients. Finally, the majority of studies (26/30) used bronchial artery embolization for managing massive hemoptysis. Despite the common methods applied in all these investigations, the different tools, materials, and techniques applied in each investigation are of interest. These are presented in Table 1.
Table 1.
Characteristics of included studies.
| Study ID | Total Subjects (% Male/ Median Age) |
Study Type | Management of Hemoptysis | Technical Success Rate | Clinical Success Rate |
|---|---|---|---|---|---|
| Abid et al., 2021 [74] | 46 (78.2/54.8) | ORCS | BAE | 97.5 | 95 |
| Yang et al., 2022 [73] | 15 (80/55.9) | ORCS | Dual-vessel intervention | 80 | 66.7 |
| Clements et al., 2022 [72] | 3 | ORCS | BAE | 100 | 100 |
| Mishra et al., 2018 [71] | 52 (82.6/48) | ORCS | BAE | NM | 92 |
| Silveira et al., 2021 [70] | 17 (47/25) | ORCS | BAE | 100 | NM |
| Seyyedi et al., 2019 [69] | 189 (58.3/NM) | ORCS | BAE | NM | 97.3 |
| Fu et al., 2019 [68] | 24 | ORCS | BAE | 94.9 | 91.7 |
| Ando et al., 2017 [67] | 35 | ORCS | BAE | 94.3 | 97 |
| Kolu et et al., 2022 [66] | 48 (68.7/52) | ORCS | BAE | 97.9 | 93.7 |
| Mehta et al., 2020 [65] | 12 | ORCS | Customized endobronchial silicone blocker | 92.3 | 92.3 |
| Keshmiri et al., 2020 [64] | 153 (68/55) | ORCS | BAE | 100 | NM |
| Marcelin et al., 2018 [63] | 19 (78.9/60.3) | ORCS | BAE | 73.7 | 84.2 |
| Miyano et al., 2017 [62] | 179 | ORCS | BAE | 93.2 | 92.8 |
| Xiaobing et al., 2022 [61] | 187 | ORCS | BAE | 100 | 86.6 |
| Han et al., 2019 [60] | 84 | ORCS | BAE | 98.9 | 82.1 |
| Seki et al., 2017 [59] | 10 | ORCS | BAE | 100 | 100 |
| Al-Samkari et al., 2019 [58] | 21 | ORCS | Antifibrinolytic agents | NM | 50 |
| Floridi et al., 2022 [57] | 13 (NM/NM) | ORCS | BAE | 94.7 | 92.3 |
| Kucukay et al., 2018 [56] | 174 (45.9/39.4) |
ORCS | BAE | 100 | 91.9 |
| Lal et al., 2021 [55] | 27 (NM/ 41.4) | ORCS | Percutaneous transthoracic embolisation | 93.1 | 88.9 |
| Lee et al., 2022 [54] | 19 | ORCS | BAE | 94.7 | 89.5 |
| Frood et al., 2020 [53] | 68 (60.2/53) | ORCS | BAE | 90 | 86.5 |
| Flight et al., 2017 [52] | 27 | ORCS | BAE | ||
| Springer et al., 2018 [51] | 30 (NM/33.5) | ORCS | BAE | NM | 95.75 |
| Le-Jun et al., 2020 [50] | 5 | ORCS | BAE | 100 | 100 |
| Ishikawa et al., 2017 [49] | 489 (46.4/69) | ORCS | BAE | 93.4 | 92 |
| Ando et al., 2019 [48] | 41 | ORCS | BAE | NM | 92.7 |
| An et al., 2023 [47] | 17 | ORCS | BAE | 100 | 100 |
| Li et al., 2023 [46] | 105 (64/NM) | ORCS | BAE | NM | 84.8 |
| Cheng et al., 2023 [45] | 127 (Group E: 47/58, Group G: 53/60) | ORCS | BAE using Embospheres alone and Embospheres with gelfoam particles |
92.99 & 97.14 | 85.96 & 97.14 |
Abbreviations: ORCS: observational retrospective cohort study, BAE: bronchial artery embolization.
3.2.2. Findings of the Studies
Some studies used techniques that differed greatly or slightly from bronchial artery embolization or used the classical technique of bronchial artery embolization with various variations depending on the circumstances and characteristics of the patients. Kucukay et al. [56] used large-sized (700–900 lm) tris-acryl microspheres (Embospheres) for bronchial embolization. The results showed the great clinical success of this technique, with very high long-term success rates. Specifically, clinical success reached 100%. The disadvantage of this technique is that the microspheres, due to their large size, can occlude the bronchial artery, which is much more central to the lesion. However, the use of a microcatheter significantly reduces the likelihood of this happening.
Xiaobing et al. and Seki et al. [59,61] used chemotherapeutic substances as means of embolization of the coronary arteries. This variant is called bronchial artery chemoembolization and seems to be advantageous over the classic technique with respect to the long-term results of treating hemoptysis due to lung cancer. It requires repeated courses of treatment, and various substances, such as epirubicin, nedaplatin, and etoposide, are used. The trophic arteries of the tumor are embolized, and it seems to be a technique that solves the problem of the difficult management of hemoptysis in cancer patients. This difficulty is due to the different and complex pathophysiology of hemoptysis in lung cancer. BAE, on the other hand, does not manage the tumor but only the hemoptysis, leading to the high recurrence rates in these patients.
In cases of anomalies, such as pseudoaneurysms, Marcelin et al. [63] suggested the use of pulmonary embolization versus bronchial embolization or endoprosthesis placement. This technique scored a high success rate relative to the difficulty and severity of the condition, while two patients died due to massive bleeding. The stent ensures the patency of the vessel; however, a complication is its occlusion due to the stent.
Mehta et al. [65] evaluated the use of customized endobronchial silicone blockers for the management of massive hemoptysis in cases where BAE is not an option. With this technique, a customized endobronchial silicone blocker is used to wedge and occlude the bleeding, with quite favourable results. The clinical success rate reached 92.3%, making this technique successful enough to replace BAE where it is not possible. Its only flaw is that it has not been studied as well as BAE to ensure its safety and success. Yang et al. [73] used the dual-vessel intervention (DVI) technique. This is a less successful technique that scored technical and clinical rates of 80% and 66.7%, respectively. This technique includes bronchial and pulmonary artery embolization with a hyperselective catheter and the use of PVA and a spring coil. Finally, the lowest clinical success rate was achieved in Samkari et al. [58], who used antifibrinolytic agents to manage hemoptysis. However, this percentage was also affected by the population group, as it included patients with cystic fibrosis, who have high failure rates in operations.
An et al. [47] examined bronchial artery embolization with a specific opening, namely, the opening of the lower wall of the aortic arch. This study reports that this method was particularly effective, with fewer complications and rebleeding, and involved the use of the JL4 catheter. The use of this catheter is particularly important, as operations of this type have not been as successful as operations using other catheters. The advantages of this catheter are found in its shape, its rigidity, and the absence of the need to create a loop.
Finally, Cheng et al. [45] analyzed the safety and effectiveness of the use of embospheres alone or in combination with gelfoam particles for BAE. The results showed that the combination of these gave significantly better results, with technical and clinical success scores of 97.14% and 97.14%, respectively. The use of pure embospheres scored technical and clinical success rates of 92.99% and 85.96%, respectively. Therefore, the major difference between these two is found in the clinical success of the two techniques. For this reason, the authors suggest the use of embospheres together with gelfoam particles to avoid clinical failure.
Etiology of Massive Hemoptysis
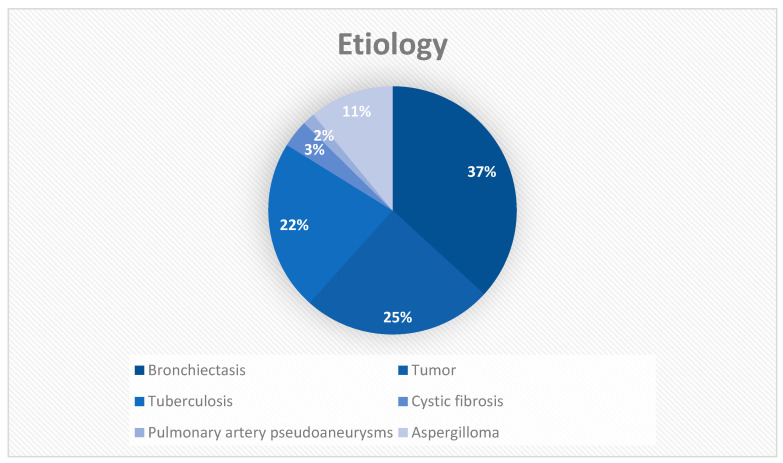
In the studies included in this systematic review, bronchiectasis is reported as the first cause, in 658 reported cases out of 2647 in total, thus covering a quarter of the causes of massive bleeding (Table 2 and Figure 2). This is followed by tumors and tuberculosis, comprising 444 and 397 cases, respectively. Cases of aspergilloma and cystic fibrosis are also common and require special management.
Table 2.
Etiology of massive hemoptysis in the studies included in this systematic review.
| Etiology of Massive Hemoptysis | Number of Incidents |
|---|---|
| Bronchiectasis | 658 |
| Tumor | 444 |
| Tuberculosis | 397 |
| Cystic fibrosis | 64 |
| Pulmonary artery pseudoaneurysms | 29 |
| Aspergilloma | 196 |
Figure 2.
Etiology of massive hemoptysis.
Radiological and Angiographic Findings
In the studies included in this systematic review, 460 cases of hypertrophy and dilatation, 44 cases of parenchymal hypervascularity, 145 cases of parenchymal blush, 73 cystic findings, 37 cavities, and 78 aneurysms were reported (Table 3). However, these numbers underestimate the actual findings because many of the studies did not report the number of findings included in the overall result.
Table 3.
Radiological and angiographic findings.
| Angiographic Findings | Incidence |
|---|---|
| Hypertrophy and dilatation | 460 |
| Parenchymal hypervascularity | 44 |
| Parenchymatous blush | 145 |
| Cystic lesion | 73 |
| Cavity | 37 |
| Aneurysmal lesion | 78 |
Recurrence, Complications, and Mortality
Table 4 presents the main postoperative complications, rebleedings, and deaths that were recorded. The main complication was chest pain, sometimes mild and sometimes more severe, which required investigation and treatment with analgesics. At least 140 such cases were reported in the studies included in this systematic review. The next most-frequent complication was fever (52 cases reported), but no further details were available from the studies regarding its cause. Only 11 cases of dysphagia and throat discomfort were reported, while even fewer, i.e., 2, were cases of paresis, one of the serious complications of the operation. A total of 149 more complications were reported, including nausea/vomiting (the most common), headache, and abdominal pain, while some of the serious and life-threatening complications reported were aortic dissection, cerebellar infarctions, and hematomas.
Table 4.
Recurrence, complications, and mortality.
| Study ID | Chest Discomfort | Fever | Dysphagia and Throat Discomfort | Paresis | Other Complications |
Recurrence | Mortality |
|---|---|---|---|---|---|---|---|
| Abid et al., 2021 [74] | 2 | 0 | 0 | 0 | 1 (headache) | 6/46 | 1 |
| Yang et al., 2022 [73] | 6 | 0 | 0 | 0 | 12 (ventricular arrhythmia) | 2/15 | 1 |
| Clements et al., 2022 [72] | 0 | 0 | 0 | 0 | 0 | 0/3 | 0 |
| Mishra et al., 2018 [71] | 5 | 0 | 0 | 1 | 0 | 2/52 | 1 |
| Silveira et al., 2021 [70] | 0 | 0 | 0 | 0 | 0 | 10/17 | 3 |
| Seyyedi et al., 2019 [69] | 23 | 0 | 10 | 0 | 6 (subintimal dissection, and pancreatitis) | 53/189 | 20 |
| Fu et al., 2019 [68] | 6 | 2 | 0 | 0 | 1 (vagus reflex) | 2/207 | 0 |
| Ando et al., 2017 [67] | 12 | 0 | 0 | 0 | 0 | 10/35 | |
| Kolu et et al., 2022 [66] | 5 | 0 | 0 | 0 | 0 | 4/45 | 1 |
| Mehta et al., 2020 [65] | 0 | 0 | 0 | 0 | 0 | 1/13 | 0 |
| Keshmiri et al., 2020 [64] | 0 | 0 | 0 | 0 | 3 (ischemia, pulmonary infarction, and spinal complications) | 43/153 | 1 |
| Marcelin et al., 2018 [63] | 0 | 0 | 0 | 0 | 0 | 0/19 | 2 |
| Miyano et al., 2017 [62] | 0 | 0 | 0 | 0 | 1 (aortic dissection) | 12/179 | 3 |
| Xiaobing et al., 2022 [61] | 34 | 38 | 0 | 0 | 87 (nausea/vomiting, increase in AST/ALT, decrease in platelets, abdominal pain) | NM | 15 |
| Han et al., 2019 [60] | 0 | 0 | 0 | 0 | 0 | 20/84 | 2 |
| Seki et al., 2017 [59] | 1 | 0 | 0 | 0 | 1 (allergic reaction) | 3/10 | 2/10 |
| Al-Samkari et al., 2019 [58] | NM | NM | NM | NM | NM | NM | NM |
| Floridi et al., 2022 [57] | NM | NM | NM | NM | NM | NM | NM |
| Kucukay et al., 2018 [56] | 9 | NM | NM | NM | NM | 14/174 | 0 |
| Lal et al., 2021 [55] | 0 | 0 | 0 | 0 | 1 (pneumothorax) | 1/29 | 0 |
| Lee et al., 2022 [54] | 0 | 0 | 0 | 0 | 0 | 7/19 | 0 |
| Frood et al., 2020 [53] | 0 | 0 | 0 | 1 | 2 (cardio-pulmonary arrest, cerebellar infarction) | NM | NM |
| Flight et al., 2017 [52] | 14 | 0 | 0 | 0 | 15 (paraesthesia, headache) | 31/49 | 5 |
| Springer et al., 2018 [51] | 0 | 1 | 0 | 0 | 3 (1 haematoma at the site of microcatheter insertion and 2 vomiting) |
10/30 | 6 |
| Le-Jun et al., 2020 [50] | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ishikawa et al., 2017 [49] | NM | NM | NM | NM | 8 (1 aortic dissection, 2 symptomatic cerebellar infarctions, and 5 mediastinal haematoma cases) | 57/489 | 23 |
| Ando et al., 2019 [48] | NM | NM | NM | NM | NM | 21/41 | NM |
| An et al., 2023 [47] | 6 | 5 | 1 | 0 | 0 | 3/17 | 0 |
| Li et al., 2023 [46] | 8 | 1 | 0 | 0 | 1 (nausea) | 29/64 | NM |
| Cheng et al., 2023 [45] | 9 | 5 | 0 | 0 | 7 (2 vomiting, 2 poor appetite, 2 allergy, 1 transient cortical blindness) |
NM | 3 |
| TOTAL | 140 | 52 | 11 | 2 | 149 | 341/1979 | 89 |
Regarding the occurrence of rebleeding, 341 cases occurred out of 1979 cases, while it is not known what happened to the remaining 668 cases due to a lack of data. These cases of rebleeding, however, were recorded at different follow-up times, that is, some appeared in the first month after the operation, others in the first six months, others in the first year, and so on. A total of 89 deaths related to hemoptysis were also recorded, which highlights the seriousness and danger of the condition, as well as the need for proper and effective treatment.
3.3. Quality Assessment
The results from the quality assessment using the Newscastle–Ottawa Scale are listed in Table 5. The included studies did not use non-exposed cohorts and were not given a star in the selection domain.
Table 5.
Quality assessment of included studies.
| Study ID | Selection | Comparability | Outcome | Total | Quality | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the Exposed Cohort | Selection of the Non-Exposed Cohort | Ascertainment of Exposure | Demonstration That Outcome of Interest Was Not Present at Start of Study | Comparability of Cohorts on the Basis of the Design or Analysis Controlled for Confounders | Assessment of Outcome | Follow-Up Long Enough for Outcomes to Occur | Adequacy of Follow-Up of Cohorts | |||
| Yang et al., 2022 [73] | * | * | * | ** | * | * | * | 8/9 | Good | |
| Clements et al., 2022 [72] | * | * | * | ** | * | * | * | 8/9 | Good | |
| Mishra et al., 2018 [71] | * | * | * | ** | * | * | * | 8/9 | Good | |
| Silveira et al., 2021 [70] | * | * | * | ** | * | * | * | 8/9 | Good | |
| Seyyedi et al., 2019 [69] | * | * | * | ** | * | * | * | 8/9 | Good | |
| Fu et al., 2019 [68] | * | * | * | ** | * | * | * | 8/9 | Good | |
| Ando et al., 2017 [67] | * | * | * | ** | * | * | * | 8/9 | Good | |
| Kolu et et al., 2022 [66] | * | * | * | ** | * | * | * | 8/9 | Good | |
| Mehta et al., 2020 [65] | * | * | * | ** | * | * | * | 8/9 | Good | |
| Keshmiri et al., 2020 [64] | * | * | * | ** | * | * | * | 8/9 | Good | |
| Marcelin et al., 2018 [63] | * | * | * | ** | * | * | * | 8/9 | Good | |
| Miyano et al., 2017 [62] | * | * | * | ** | * | * | * | 8/9 | Good | |
| Xiaobing et al., 2022 [61] | * | * | * | ** | * | * | * | 8/9 | Good | |
| Han et al., 2019 [60] | * | * | * | ** | * | * | * | 8/9 | Good | |
| Seki et al., 2017 [59] | * | * | * | ** | * | * | * | 8/9 | Good | |
| Al-Samkari et al., 2019 [58] | * | * | * | ** | * | * | * | 8/9 | Good | |
| Floridi et al., 2022 [57] | * | * | * | ** | * | * | * | 8/9 | Good | |
| Kucukay et al., 2018 [56] | * | * | * | ** | * | * | * | 8/9 | Good | |
| Lal et al., 2021 [55] | * | * | * | ** | * | * | * | 8/9 | Good | |
| Lee et al., 2022 [54] | * | * | * | ** | * | * | * | 8/9 | Good | |
| Frood et al., 2020 [53] | * | * | * | ** | * | * | * | 8/9 | Good | |
| Flight et al., 2017 [52] | * | * | * | ** | * | * | * | 8/9 | Good | |
| Springer et al., 2018 [51] | * | * | * | ** | * | * | * | 8/9 | Good | |
| Le-Jun et al., 2020 [50] | * | * | * | ** | * | * | * | 8/9 | Good | |
| Ishikawa et al., 2017 [49] | * | * | * | ** | * | * | * | 8/9 | Good | |
| Ando et al., 2019 [48] | * | * | * | ** | * | * | * | 8/9 | Good | |
| An et al., 2023 [47] | * | * | * | ** | * | * | * | 8/9 | Good | |
| Li et al., 2023 [46] | * | * | * | ** | * | * | * | 8/9 | Good | |
| Cheng et al., 2023 [45] | * | * | * | ** | * | * | * | 8/9 | Good | |
| Abid et al., 2021 [74] | * | * | * | ** | * | * | * | 8/9 | Good | |
With *, the star given to each category that excludes the risk of bias is denoted. Each category is evaluated with a blank or a *, except for the Comparability category which is evaluated with a blank or a * or **.
3.4. Meta-Analysis Results
A proportional meta-analysis was performed for all studies that used a common mode of management of massive hemoptysis, that is, BAE, and provided relevant data [45,46,47,48,49,50,51,52,53,54,56,57,59,60,61,62,63,64,66,68,69,70,71,72,74].
3.4.1. Technical Success Meta-Analysis
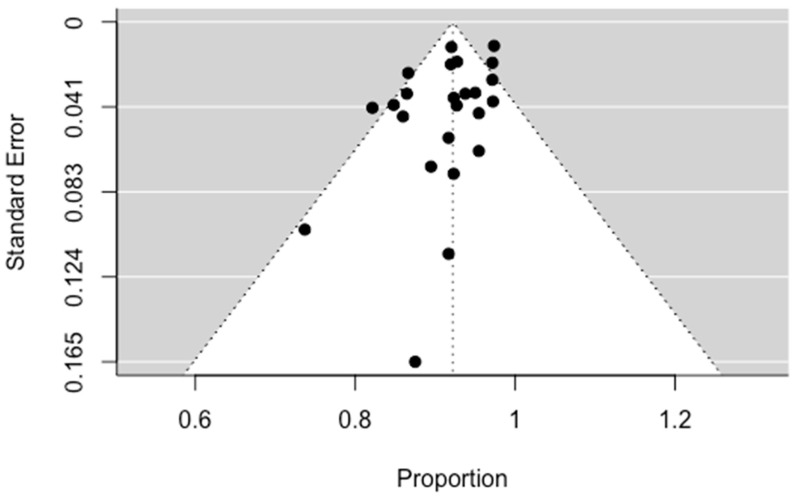
The results of the statistical analysis showed a pooled technical success rate of 97.22%, confirming the technical success of the operation. Technical success is defined as successful embolization of the bronchial arteries. These results are presented with a forest plot in Figure 3. Significant heterogeneity between the included studies was observed (I2 = 77%, p < 0.001). Publication bias for technical success presented significant asymmetry (Figure 4, p = 0.0024).
Figure 3.
Forest plot for technical success [45,47,49,50,53,54,56,57,59,60,61,62,63,64,66,67,68,70,72,74].
Figure 4.
Funnel plot for technical success.
3.4.2. Clinical Success
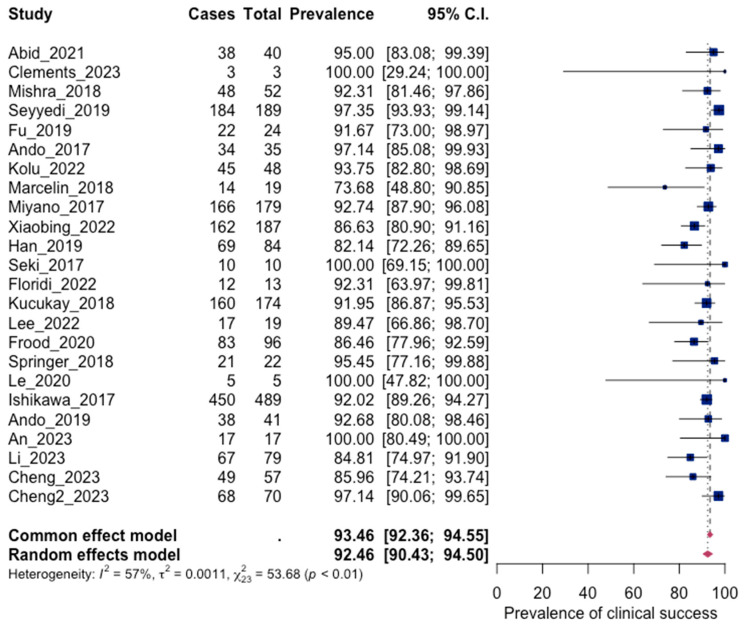
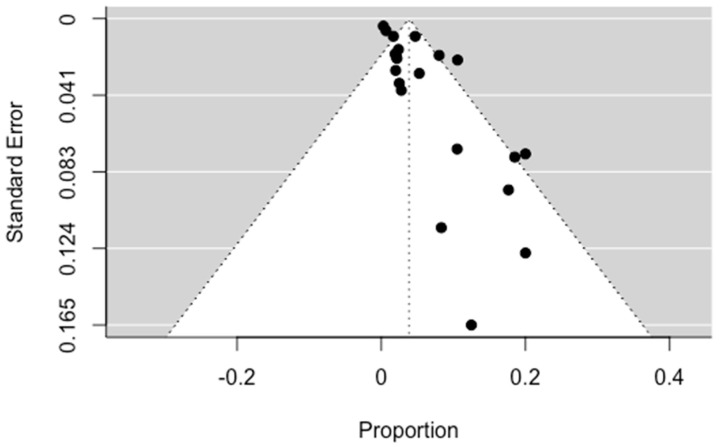
Clinical success is defined as complete cessation of hemoptysis after bronchial arterial embolization. The statistical analysis of the results of 24 studies showed a pooled clinical success equal to 92.46% (90.43; 94.50 95% CI), with moderate heterogeneity (I2: 57%, p < 0.001). These results confirm the effectiveness of BAE in treating massive hemoptysis. No significant asymmetry was observed in the funnel plot regarding publication bias (p = 0.0772). The results are presented in Figure 5 and Figure 6.
Figure 5.
Forest plot for clinical success [45,46,47,48,49,50,51,53,54,56,57,59,60,61,62,63,66,67,68,69,71,72,74].
Figure 6.
Funnel plot for clinical success.
3.4.3. Recurrence
All episodes of recurrence and rebleeding recorded in the studies in this systematic review during follow-up were used to calculate the prevalence of recurrence. The pooled recurrence was calculated to be equal to 21.46% (14.04; 28.89 95% CI), which shows the frequent occurrence of recurrence in patients, which can often be life-threatening. The result was considered statistically significant, with p < 0.0001, while high heterogeneity was also distinguished. These results are visible in Figure 7 and Figure 8, below.
Figure 7.
Forest plot for recurrence [46,47,48,49,50,51,52,54,56,59,60,62,63,64,66,67,68,69,70,71,72,74].
Figure 8.
Regression test for funnel plot asymmetry. Significant asymmetry was observed (p = 0.0246).
3.4.4. Mortality
The pooled result for mortality was 3.5% (95% CI: 1.78; 5.21). Mortality, although it is a rare complication of massive hemoptysis, is a real risk that has not yet been eliminated. Significant asymmetry was observed in the funnel plot, indicating publication bias (p = 0.0002). The results are presented in Figure 9 and Figure 10.
Figure 9.
Forest plot for mortality [45,47,49,50,51,52,54,56,59,60,61,62,63,64,66,68,69,70,71,72,74].
Figure 10.
Funnel plot for mortality.
4. Discussion
In this systematic review, 30 studies related to the management of massive hemoptysis were included, of which 26 used bronchial artery embolization as a management method [45,46,47,48,49,50,51,52,53,54,56,57,59,60,61,62,63,64,66,67,68,69,70,71,72,74]. The 30 studies were observational retrospective cohort studies. As a result, no definitive conclusions can be made about the success of the methods for treating massive hemoptysis. The technical success of BAE ranges from 73.7% to 100%, while the clinical success ranges from 82.1% to 100%. This large variation depends on various factors, such as the technique and agents used, as well as the cause of the hemoptysis. Regarding the techniques, these initially include access to the vascular tree of the pulmonary parenchyma. This usually occurs via the femoral artery, although the transaxillary route has also been used. Embolization of the visible abnormal arteries then follows. The choice of the appropriate embolization factors is also important, as it has been found that they affect the success of the operation [35,36,37,75,76]. The most common agent is polyvinyl alcohol; however, a combination of agents has been found to slightly increase the technical success but significantly increase the clinical success [35,36,37,75,76]. Cheng et al. [45] showed that with the combination of embospheres and gelfoam particles, the technical success increased by 4.18%, while the clinical success jumped from 85.96% (without gelfoam particles) to 97.14%. It is worth mentioning the value of superselective arterial catheterization, which achieves the best results with the fewest possible complications [35,36,37,75,76].
The results of the meta-analysis demonstrate the success of BAE in the treatment of massive hemoptysis. Specifically, BAE scored a pooled technical success equal to 97.22% as well as a pooled clinical success equal to 92.46%. Therefore, BAE can with great ability both achieve arterial embolization and interrupt massive hemoptysis. However, the level of recurrence seems to be high (21.46%). Therefore, despite the immediate success of the intervention, the long-term success is limited due to the high recurrence numbers. Finally, mortality is limited to 3.5%, which also confirms the success of the intervention.
Surgical treatment is an alternative to BAE, which, however, is not minimally invasive, instead requiring a long operation with lobectomy of the bleeding lung segment. It is chosen in patients in whom temporary hemostasis or BAE will not produce a long-term effect, leading to rebleeding in a short period of time. It is a prerequisite that the patient is cardiorespiratorily fit for such an operation and that the bleeding is unilateral. Etiological therapy is chosen for respiratory diseases that do not cause anatomical damage and must be accompanied by respiratory support by administering oxygen [10,11,18]. It is the method of choice for conditions such as aspergilloma as well as for iatrogenic injuries that cause massive hemoptysis [77,78].
In the current systematic review, we defined massive hemoptysis as the expulsion of blood through the oral route in an amount exceeding 200 mL per twenty-four hours. In smaller amounts, hemoptysis is categorized as mild or moderate. In these cases, the patient is usually hemodynamically stable and the way to deal with hemoptysis changes, including treatment of the etiology that causes hemoptysis, and thus, together with the treatment of the cause of the hemoptysis, the hemoptysis itself is removed. However, it has been found that hemoptysis can be treated in these cases with the use of nebulized tranexamic acids. These are synthetic analogues of lysine with anti-fibrinolytic action. Specifically, they cause the suspension activation of plasminogen to plasmin and block the action of plasmin on fibrin. In the double-blind, randomized study by Wand et al., they were found to score 96% in the treatment of hemoptysis versus placebo, which achieved only 50%. This confirms the success of TAs in the management of mild-to-moderate hemoptysis. Also, the study showed that co-administration of antibiotics with TA does not increase the success of the intervention [14,79,80,81,82].
However, it has been found that moderate hemoptysis can potentially have the same prognosis of rebleeding as massive hemoptysis as well as the same mortality. For this reason, it is suggested that it be treated in the same way as the massive one. In other words, it is recommended to use BAE to treat it rather than etiological treatment or TA. In terms of success rates, these show the significant ability of BAE to manage moderate hemoptysis, scoring more than 96% and 94% for technical and clinical success, respectively [14,79,80,81].
Many factors have been implicated as risk factors for hemoptysis in the population. Initially, it was found that bronchiectasis is associated with an increased risk of hemoptysis, as well as hypertrophic bronchial arteries that are associated with heavier and faster hemodynamic instability [6,9,16,17,83,84]. As for hypertrophic arteries, it has been found that these are located in patients carrying the mutated BMPR2 gene. However, this mutation is not associated with an increased risk of hemoptysis but only with an increased frequency of bronchial artery hypertrophy. Smoking also increases the risk of developing hemoptysis, while anticoagulants and antiplatelet drugs increase both the risk of hemoptysis and the severity of this hemoptysis. Diabetes was also implicated as a risk factor, perhaps due to the vascular damage it causes. Hemoptysis occurs more often in males, while it occurs less often in diseases with a course between 1 and 5 years. Finally, with regard to massive hemoptysis, this occurs more often in diseases that affect two lobes or more, while the lower left lobe is less often involved in episodes of massive hemoptysis [6,8,9,11,83,84,85,86].
It has been found that, over time, the rate of clinical success decreases significantly. From the very first month after BAE, the clinical success rate can be significantly reduced due to the resulting rebleeding cases, which can be fatal. For this reason, long-term follow-up is considered useful in order to monitor the course of the patient’s disease. In particular, certain conditions are associated with an increased risk of rebleeding in more than half of patients. Such diseases are bronchiectasis, aspergilloma, and especially lung tumors (primary and metastatic). In these cases, surgical treatment of hemoptysis should be discussed to reduce the risk of rebleeding. Many times, during follow-up, the need for re-interventions can be seen [45,46,47,48,49,50,51,52,53,54,56,57,59,60,61,62,63,64,66,67,68,69,70,71,72,74].
Rebleeding after BAE is the most frequent complication of surgery and determines the prognosis, as it can be fatal. Rebleeding occurs most frequently after the first month to the first year, with a mean day of occurrence of 293 postoperative days. It next most frequently appears in the first month after BAE. Rarely, however, rebleeding can occur after one year of BAE. However, these numbers vary quite a bit and are determined by the existence or not of risk factors. Such factors are diabetes, aspergilloma, and the existence of an arteriovenous shunt. In particular, with regard to aspergilloma, rebleeding can reach 100%, emphasizing the need for regular follow-up and discussion of surgical lobectomy if the patient is deemed cardiorespiratorily fit for such an operation [45,46,47,48,49,50,51,52,53,54,56,57,59,60,61,62,63,64,66,67,68,69,70,71,72,74].
Paresis, a serious complication, was reported twice in the included studies in this systematic review. Mishra et al. [71] documented monoparesis of the right lower extremity after BAE. This paresis was blamed on a small focal hyperintensity of the spinal cord due to a possible embolism of the spinal artery, as confirmed by magnetic resonance imaging. This complication requires physiotherapy that leads to partial-to-complete restoration of the damage. Other serious complications reported include aortic dissection, which occurred in one incident due to a fragile aortic wall. Two cases of cerebral infarction were also reported, one of which was attributed to infarction arising from the vertebral artery. This emphasizes the need for special care when embolizing near the vertebral artery.
Further investigation needs to be undertaken to find ways to treat massive hemoptysis non-invasively. Also, further investigation is required into how to prevent the complications of BAE, such as rebleeding, which occurs at a very high rate in some cases.
This research has certain limitations. One of them concerns the type of studies that were selected, these being retrospective observational cohort studies and not randomized controlled trials. This is due to the non-existence of other types of studies. Another constraint is the restriction of studies for inclusion to those written in the English language, which created publication bias, as well as the literature search being limited to a narrow period of time. Our systematic review does, however, have several strengths. Firstly, there is the rigorous methodology used according to the PRISMA guidelines and the exploration and reporting of all possible treatments of massive hemoptysis, in addition to only recent studies, i.e., those published in the last six years, having been selected.
5. Conclusions
In conclusion, massive hemoptysis is a life-threatening condition and is a frequent symptom of various respiratory diseases, such as COVID-19. Its treatment can be achieved in terms of technical and clinical success with the use of bronchial artery embolization. However, when this method is not indicated, the treatment of massive bleeding is achieved by using other equally effective methods. Complications appear in a large number of patients, the most common of which is chest discomfort. Rebleeding is a major problem, with massive bleeding occurring frequently, which can be fatal. Finally, mortality from massive hemoptysis occurs in a low percentage of patients, which reinforces the effectiveness of the methods of dealing with massive hemoptysis.
Author Contributions
Conceptualization, E.K. (Eleni Karlafti), L.K., G.T., G.K., C.S., A.M. and D.P.; methodology, E.K. (Evangelia Kotzakioulafi); software, D.T.; validation, E.K. (Eleni Karlafti), D.T., E.K. (Evangelia Kotzakioulafi) and S.N.; formal analysis, E.K. (Evangelia Kotzakioulafi) and D.T.; investigation, E.K. (Eleni Karlafti), D.T. and S.N.; resources, D.P., A.M. and C.S.; data curation, D.T. and S.N.; writing—original draft preparation, D.T., E.K. (Eleni Karlafti) and E.K. (Evangelia Kotzakioulafi); writing—review and editing, E.K. (Eleni Karlafti), D.T., E.K. (Evangelia Kotzakioulafi), L.K., G.T., G.K., C.S., A.M. and D.P.; visualization, E.K. (Evangelia Kotzakioulafi) and D.T.; supervision, C.S., G.K., A.M. and D.P.; project administration, C.S., G.K., A.M. and D.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data are created.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Pan C.X., Palathra B.C., Leo-To W.F. Management of Respiratory Symptoms in Those with Serious Illness. Med. Clin. N. Am. 2020;104:455–470. doi: 10.1016/j.mcna.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Umakanthan S., Sahu P., Ranade A.V., Bukelo M.M., Rao J.S., Lf A.-M., Dahal S., Kumar H., Kv D. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19) Postgrad Med. J. 2020;96:753–758. doi: 10.1136/postgradmedj-2020-138234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pradhan M., Shah K., Alexander A., Ajazuddin, Minz S., Singh M.R., Singh D., Yadav K., Chauhan N.S. COVID-19: Clinical presentation and detection methods. J. Immunoass. Immunochem. 2022;43:1951291. doi: 10.1080/15321819.2021.1951291. [DOI] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dudha M., Lehrman S., Aronow W.S., Rosa J. Hemoptysis: Diagnosis and treatment. Compr. Ther. 2009;35:139–149. [PubMed] [Google Scholar]
- 6.Liippo K., Vasankari T. Hemoptysis. Duodecim. 2011;127:178–184. [PubMed] [Google Scholar]
- 7.Xi Y., Liu D., Yang C., Wu X., Nong L., He W., Liu X., Li Y. Cause of massive hemoptysis in critical patients and the effect of bronchial artery embolization. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2018;30:671–676. doi: 10.3760/cma.j.issn.2095-4352.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Noseworthy T.W., Anderson B.J. Massive hemoptysis. CMAJ. 1986;135:1097–1099. [PMC free article] [PubMed] [Google Scholar]
- 9.Prey B., Francis A., Williams J., Krishnadasan B. Evaluation and Treatment of Massive Hemoptysis. Surg. Clin. N. Am. 2022;102:465–481. doi: 10.1016/j.suc.2021.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Ittrich H., Bockhorn M., Klose H., Simon M. The Diagnosis and Treatment of Hemoptysis. Dtsch. Arztebl. Int. 2017;114:371–381. doi: 10.3238/arztebl.2017.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Earwood J.S., Thompson T.D. Hemoptysis: Evaluation and management. Am. Fam. Physician. 2015;91:243–249. [PubMed] [Google Scholar]
- 12.Aidé M.A. Hemoptysis. J. Bras Pneumol. 2010;36:278–280. doi: 10.1590/S1806-37132010000300002. [DOI] [PubMed] [Google Scholar]
- 13.DiLeo M.D., Amedee R.G., Butcher R.B. Hemoptysis and pseudohemoptysis: The patient expectorating blood. Ear Nose Throat J. 1995;74:822–824+826+828. doi: 10.1177/014556139507401209. [DOI] [PubMed] [Google Scholar]
- 14.Gavelli F., Patrucco F., Statti G., Balbo P.E. Mild-to-moderate hemoptysis: A diagnostic and clinical challenge. Minerva Med. 2018;109:239–247. doi: 10.23736/S0026-4806.18.05576-3. [DOI] [PubMed] [Google Scholar]
- 15.Larici A.R., Franchi P., Occhipinti M., Contegiacomo A., del Ciello A., Calandriello L., Storto M.L., Marano R., Bonomo L. Diagnosis and management of hemoptysis. Diagn. Interv. Radiol. 2014;20:299–309. doi: 10.5152/dir.2014.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bidwell J.L., Pachner R.W. Hemoptysis: Diagnosis and management. Am. Fam. Physician. 2005;72:1253–1260. [PubMed] [Google Scholar]
- 17.Khalil A., Fedida B., Parrot A., Haddad S., Fartoukh M., Carette M.-F. Severe hemoptysis: From diagnosis to embolization. Diagn. Interv. Imaging. 2015;96:775–788. doi: 10.1016/j.diii.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Jin F., Li Q., Bai C., Wang H., Li S., Song Y., Zeng Y., Zhou R., Li W., Hu C., et al. Chinese Expert Recommendation for Diagnosis and Treatment of Massive Hemoptysis. Respiration. 2020;99:83–92. doi: 10.1159/000502156. [DOI] [PubMed] [Google Scholar]
- 19.Hsiao E.I., Kirsch C.M., Kagawa F.T., Wehner J.H., Jensen W.A., Baxter R.B. Utility of fiberoptic bronchoscopy before bronchial artery embolization for massive hemoptysis. Am. J. Roentgenol. 2001;177:861–867. doi: 10.2214/ajr.177.4.1770861. [DOI] [PubMed] [Google Scholar]
- 20.Sakr L., Dutau H. Massive hemoptysis: An update on the role of bronchoscopy in diagnosis and management. Respiration. 2010;80:38–58. doi: 10.1159/000274492. [DOI] [PubMed] [Google Scholar]
- 21.Cahill B.C., Ingbar D.H. Massive hemoptysis. Assessment and management. Clin. Chest Med. 1994;15:147–167. doi: 10.1016/S0272-5231(21)01061-3. [DOI] [PubMed] [Google Scholar]
- 22.Cordovilla R., de Miguel E.B., Ares A.N., Povedano F.J.C., Ortega I.H., Merchán R.J. Diagnóstico y tratamiento de la hemoptisis. Arch Bronconeumol. 2016;52:368–377. doi: 10.1016/j.arbres.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Wolfe J.D., Simmons D.H. Hemoptysis: Diagnosis and management. West J. Med. 1977;127:383–390. [PMC free article] [PubMed] [Google Scholar]
- 24.Revel M.P., Fournier L.S., Hennebicque A.S., Cuenod C.A., Meyer G., Reynaud P., Frija G. Can CT replace bronchoscopy in the detection of the site and cause of bleeding in patients with large or massive hemoptysis? Am. J. Roentgenol. 2002;179:1217–1224. doi: 10.2214/ajr.179.5.1791217. [DOI] [PubMed] [Google Scholar]
- 25.Yoon W., Kim Y.H., Kim J.K., Kim Y.C., Park J.G., Kang H.K. Massive Hemoptysis: Prediction of Nonbronchial Systemic Arterial Supply with Chest CT. Radiology. 2003;227:232–238. doi: 10.1148/radiol.2271020324. [DOI] [PubMed] [Google Scholar]
- 26.Solomonov A., Fruchter O., Zuckerman T., Brenner B., Yigla M. Pulmonary hemorrhage: A novel mode of therapy. Respir. Med. 2009;103:1196–1200. doi: 10.1016/j.rmed.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Colchen A., Fischler M. Emergency interventional bronchoscopies. Rev. Pneumol. Clin. 2011;67:209–213. doi: 10.1016/j.pneumo.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Jean-Baptiste E. Clinical assessment and management of massive hemoptysis. Crit. Care Med. 2000;28:1642–1647. doi: 10.1097/00003246-200005000-00066. [DOI] [PubMed] [Google Scholar]
- 29.Corey R., Hla K.M. Major and massive hemoptysis: Reassessment of conservative management. Am. J. Med. Sci. 1987;294:301–309. doi: 10.1097/00000441-198711000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Crocco J.A., Rooney J.J., Fankushen D.S., DiBenedetto R.J., Lyons H.A. Massive hemoptysis. Arch. Intern. Med. 1968;121:495–498. doi: 10.1001/archinte.1968.03640060009002. [DOI] [PubMed] [Google Scholar]
- 31.Wahidi M.M., Rocha A.T., Hollingsworth J.W., Govert J.A., Feller-Kopman D., Ernst A. Contraindications and safety of transbronchial lung biopsy via flexible bronchoscopy. A survey of pulmonologists and review of the literature. Respiration. 2005;72:285–295. doi: 10.1159/000085370. [DOI] [PubMed] [Google Scholar]
- 32.Papin T.A., Lynch J.P., Weg J.G. Transbronchial biopsy in the thrombocytopenic patient. Chest. 1985;88:549–552. doi: 10.1378/chest.88.4.549. [DOI] [PubMed] [Google Scholar]
- 33.Ernst A., Eberhardt R., Wahidi M., Becker H.D., Herth F.J.F. Effect of routine clopidogrel use on bleeding complications after transbronchial biopsy in humans. Chest. 2006;129:734–737. doi: 10.1378/chest.129.3.734. [DOI] [PubMed] [Google Scholar]
- 34.Dransfield M.T., Garver R.I., Weill D. Standardized guidelines for surveillance bronchoscopy reduce complications in lung transplant recipients. J. Heart Lung Transpl. 2004;23:110–114. doi: 10.1016/S1053-2498(03)00098-6. [DOI] [PubMed] [Google Scholar]
- 35.Lorenz J., Sheth D., Patel J. Bronchial artery embolization. Semin. Interv. Radiol. 2012;29:155–160. doi: 10.1055/s-0032-1326923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panda A., Bhalla A.S., Goyal A. Bronchial artery embolization in hemoptysis: A systematic review. Diagn. Interv. Radiol. 2017;23:307–317. doi: 10.5152/dir.2017.16454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaufman C.S., Kwan S.W. Bronchial Artery Embolization. Semin. Interv. Radiol. 2022;39:210–217. doi: 10.1055/s-0042-1751293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Page M.J., Moher D., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010;36:1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 41.Balduzzi S., Rücker G., Schwarzer G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Higgins J.P.T. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Higgins J., Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022) The Cochrane Collaboration; London, UK: 2022. [Google Scholar]
- 45.Cheng L., Zhao X., Hu X., Huang J., Zhang X. Safety and Efficacy Comparison of Embospheres and Gelfoam Particles in Bronchial Artery Embolization for Massive Hemoptysis. Altern. Ther. Health Med. 2023;29:298–301. [PubMed] [Google Scholar]
- 46.Li H., Ding X., Zhai S., Gao K. A retrospective study on the management of massive hemoptysis by bronchial artery embolization: Risk factors associated with recurrence of hemoptysis. BMC Pulm. Med. 2023;23:87. doi: 10.1186/s12890-023-02371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.An J., Dong Y., Niu H. Application of the 5F JL4 Catheter in Bronchial Artery Embolization with the Opening in the Inferior Wall of the Aortic Arch. Vasc. Endovasc. Surg. 2023;57:379–385. doi: 10.1177/15385744221149910. [DOI] [PubMed] [Google Scholar]
- 48.Ando T., Kawashima M., Masuda K., Takeda K., Okuda K., Suzuki J., Ohshima N., Horibe M., Tamura A., Nagai H., et al. Exacerbation of chronic pulmonary aspergillosis was associated with a high rebleeding rate after bronchial artery embolization. Respir. Investig. 2019;57:260–267. doi: 10.1016/j.resinv.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 49.Ishikawa H., Hara M., Ryuge M., Takafuji J., Youmoto M., Akira M., Nagasaka Y., Kabata D., Yamamoto K., Shintani A. Efficacy and safety of super selective bronchial artery coil embolisation for haemoptysis: A single-centre retrospective observational study. BMJ Open. 2017;7:e014805. doi: 10.1136/bmjopen-2016-014805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Le-Jun F., Sun Y., Fan Y., Jin S. The effect of transcatheter bronchial artery embolization in five patients with bronchial artery aneurysm. Postep. Kardiol Interwencyjnej. 2020;16:330–335. doi: 10.5114/aic.2020.99269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Springer D.M., Cofta S., Juszkat R., Żabicki B., Goździk-Spychalska J., Nowicka A., Winiarska H., Batura-Gabryel H. The effectiveness of bronchial artery embolisation in patients with haemoptysis. Adv. Respir. Med. 2018;86:220–226. doi: 10.5603/ARM.2018.0035. [DOI] [PubMed] [Google Scholar]
- 52.Flight W.G., Barry P.J., Bright-Thomas R.J., Butterfield S., Ashleigh R., Jones A.M. Outcomes Following Bronchial Artery Embolisation for Haemoptysis in Cystic Fibrosis. Cardiovasc. Interv. Radiol. 2017;40:1164–1168. doi: 10.1007/s00270-017-1626-0. [DOI] [PubMed] [Google Scholar]
- 53.Frood R., Karthik S., Mirsadraee S., Clifton I., Flood K., McPherson S.J. Bronchial Artery Embolisation for Massive Haemoptysis: Immediate and Long-Term Outcomes-A Retrospective Study. Pulm. Ther. 2020;6:107–117. doi: 10.1007/s41030-020-00112-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee Y., Lee M., Hur S., Kim H.-C., Jae H.J., Chung J.W., Choi J.W. Bronchial and non-bronchial systemic artery embolization with transradial access in patients with hemoptysis. Diagn. Interv. Radiol. 2022;28:359–363. doi: 10.5152/dir.2022.201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lal A., Bansal A., Chaluvashetty S.B., Sandhu M.S., Gorsi U. Percutaneous transthoracic embolisation for massive haemoptysis secondary to peripheral pulmonary artery pseudoaneurysms. Eur. Radiol. 2021;31:2183–2190. doi: 10.1007/s00330-020-07348-w. [DOI] [PubMed] [Google Scholar]
- 56.Kucukay F., Topcuoglu O.M., Alpar A., Altay Ç.M., Kucukay M.B., Ozbulbul N.I. Bronchial Artery Embolization with Large Sized (700–900 µm) Tris-acryl Microspheres (Embosphere) for Massive Hemoptysis: Long-Term Results (Clinical Research) Cardiovasc. Interv. Radiol. 2018;41:225–230. doi: 10.1007/s00270-017-1818-7. [DOI] [PubMed] [Google Scholar]
- 57.Floridi C., Boscarato P., Ventura C., Bruno A., Rossini N., Baldassari M., Lanza C., Fabrizzi B., Candelari R., Giovagnoni A. Role of Bronchial Artery Embolization as Early Treatment Option in Stable Cystic Fibrosis Patients with Sub-Massive Hemoptysis: Personal Experience and Literature Review. J. Clin. Med. 2022;11:6432. doi: 10.3390/jcm11216432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Al-Samkari H., Shin K., Cardoni L., Pighetti E.H., Rits S., McMahon L., Perkins R., Uluer A., Connors J.M. Antifibrinolytic Agents for Hemoptysis Management in Adults with Cystic Fibrosis. Chest. 2019;155:1226–1233. doi: 10.1016/j.chest.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 59.Seki A., Shimono C. Transarterial chemoembolization for management of hemoptysis: Initial experience in advanced primary lung cancer patients. Jpn. J. Radiol. 2017;35:495–504. doi: 10.1007/s11604-017-0659-2. [DOI] [PubMed] [Google Scholar]
- 60.Han K., Yoon K.W., Kim J.H., Kim G.M. Bronchial Artery Embolization for Hemoptysis in Primary Lung Cancer: A Retrospective Review of 84 Patients. J. Vasc. Interv. Radiol. 2019;30:428–434. doi: 10.1016/j.jvir.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 61.Xiaobing L., Meipan Y., Pengfei X., Yue Z., Ying L., Xiangnan L., Yu Q., Yaozhen M., Chunxia L., Gang W. Bronchial Artery Chemoembolization for Hemoptysis in Advanced Primary Lung Cancer. Clin. Lung Cancer. 2022;23:e203–e209. doi: 10.1016/j.cllc.2021.10.011. [DOI] [PubMed] [Google Scholar]
- 62.Miyano Y., Kanzaki M., Onuki T. Bronchial artery embolization: First-line option for managing massive hemoptysis. Asian Cardiovasc. Thorac. Ann. 2017;25:618–622. doi: 10.1177/0218492316667231. [DOI] [PubMed] [Google Scholar]
- 63.Marcelin C., Soussan J., Desmots F., Gaubert J.-Y., Vidal V., Bartoli J.M., Izaaryene J. Outcomes of Pulmonary Artery Embolization and Stent Graft Placement for the Treatment of Hemoptysis Caused by Lung Tumors. J. Vasc. Interv. Radiol. 2018;29:975–980. doi: 10.1016/j.jvir.2018.01.773. [DOI] [PubMed] [Google Scholar]
- 64.Keshmiri M.S., Shafaghi S., Sharif-Kashani B., Sadoughi A., Ghorbani F., Naghashzadeh F., Abedini A. Preemptive non-selective bronchial artery angioembolization to reduce recurrence rate of hemoptysis. Multidiscip Respir. Med. 2020;15:723. doi: 10.4081/mrm.2020.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mehta R.M., Godara R., Bhat R.S., Loknath C., Singla A. A Novel Technique for the Management of Massive Hemoptysis: The Customized Endobronchial Silicone Blocker. Innovations. 2020;15:142–147. doi: 10.1177/1556984520904351. [DOI] [PubMed] [Google Scholar]
- 66.MKolu, Kurtuluş Ş., Dere O., Yurttutan N., Yıldırım I.O. Embolization with more diluted glue-lipiodol in patients with massive hemoptysis: Single center experience results. Eur. Rev. Med. Pharmacol. Sci. 2022;26:1543–1548. doi: 10.26355/eurrev_202203_28219. [DOI] [PubMed] [Google Scholar]
- 67.Ando T., Kawashima M., Masuda K., Takeda K., Okuda K., Suzuki J., Ohshima N., Matsui H., Tamura A., Nagai H., et al. Clinical and Angiographic Characteristics of 35 Patients with Cryptogenic Hemoptysis. Chest. 2017;152:1008–1014. doi: 10.1016/j.chest.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 68.Fu Z., Liang Y., Zhao W., Tian J., Cai F., Zhang X. Safety and efficacy of transcatheter embolization in patients with massive hemoptysis due to intercostal pulmonary venous shunts. Radiol. Med. 2019;124:588–594. doi: 10.1007/s11547-019-01020-0. [DOI] [PubMed] [Google Scholar]
- 69.Seyyedi S.R., Sadeghipour P., Sadr M., Shafe O., Moosavi J., Aloosh O., Abedini A., Sharif-Kashani B. Outcomes and Complications of Bronchial Angioembolization in Patients with Massive Hemoptysis. Tanaffos. 2019;18:310–314. [PMC free article] [PubMed] [Google Scholar]
- 70.Da Silveira M.A.P., da Silveira P.A.P., Beltrami F.G., Scaffaro L.A., Dalcin P.d.T.R. Clinical outcomes of cystic fibrosis patients with hemoptysis treated with bronchial artery embolization. J. Bras. Pneumol. 2021;47:e20200557. doi: 10.36416/1806-3756/e20200557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mishra A., Mathur A., Pathak K., Katoch C.D.S., Khera A. Bronchial artery embolization in treatment of hemoptysis: Treatment efficacy and complications at a tertiary care chest centre. Med. J. Armed Forces India. 2018;74:352–357. doi: 10.1016/j.mjafi.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clements W., Venn G., McGiffin D., Moriarty H.K., Joseph T., Goh G.S., Whitford H., Keating D. Chronic Thromboembolic Pulmonary Hypertension (CTEPH) and massive hemoptysis: The rationale for bronchial artery embolization. Respir. Med. 2022;195:106784. doi: 10.1016/j.rmed.2022.106784. [DOI] [PubMed] [Google Scholar]
- 73.Yang Q., Luo L.C., Wei H., Yi Q., Luo W. Dual-vessel intervention treatment for massive hemoptysis caused by lung cavitary lesions. Eur. J. Radiol. 2022;154:110448. doi: 10.1016/j.ejrad.2022.110448. [DOI] [PubMed] [Google Scholar]
- 74.Abid N., Loukil M., Mokni A., Badri I., Bouzaidi K., Ghrairi H. Outcomes of bronchial artery embolization for the management of hemoptysis. Tunis Med. 2021;99:264–268. [PMC free article] [PubMed] [Google Scholar]
- 75.Temel U., Akgul A.G., Dogan S. Bronchial Artery Embolization, an Increasingly Used Method for Hemoptysis; Treatment and Avoidance. Sisli. Etfal. Hastan. Tıp. Bul. 2020;54:313–319. doi: 10.14744/SEMB.2020.68870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burke C.T., Mauro M.A. Bronchial artery embolization. Semin. Interv. Radiol. 2004;21:43–48. doi: 10.1055/s-2004-831404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kiral H., Evman S., Tezel C., Alpay L., Lacin T., Baysungur V., Yalcinkaya I. Pulmonary resection in the treatment of life-threatening hemoptysis. Ann. Thorac Cardiovasc. Surg. 2015;21:125–131. doi: 10.5761/atcs.oa.14-00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nakajima T., Yoshino I. Massive Hemoptysis; Clinical Approach and Surgical Treatment. Kyobu Geka. 2015;68:665–670. [PubMed] [Google Scholar]
- 79.Ketai L.H., Mohammed T.-L.H., Kirsch J., Kanne J.P., Chung J.H., Donnelly E.F., Ginsburg M.E., Heitkamp D.E., Henry T.S., Kazerooni E.A., et al. ACR appropriateness criteria® hemoptysis. J. Thorac Imaging. 2014;29:W19–W22. doi: 10.1097/RTI.0000000000000084. [DOI] [PubMed] [Google Scholar]
- 80.Johnson J.L. Manifestations of hemoptysis. How to manage minor, moderate, and massive bleeding. Postgrad Med. 2002;112:101–106+108–109+113. doi: 10.3810/pgm.2002.10.1335. [DOI] [PubMed] [Google Scholar]
- 81.Lee M.K., Kim S., Yong S.J., Shin K.C., Kim H.S., Yu T., Choi E.H., Lee W. Moderate hemoptysis: Recurrent hemoptysis and mortality according to bronchial artery embolization. Clin. Respir. J. 2015;9:53–64. doi: 10.1111/crj.12104. [DOI] [PubMed] [Google Scholar]
- 82.Wand O., Guber E., Guber A., Shochet G.E., Israeli-Shani L., Shitrit D. Inhaled Tranexamic Acid for Hemoptysis Treatment. Chest. 2018;154:1379–1384. doi: 10.1016/j.chest.2018.09.026. [DOI] [PubMed] [Google Scholar]
- 83.Luo L., Luo J., Jiang Y. A retrospective analysis of risk factors for massive hemoptysis in patients with bronchiectasis. BMC Pulm. Med. 2022;22:214. doi: 10.1186/s12890-022-02006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tio D., Leter E., Boerrigter B., Boonstra A., Vonk-Noordegraaf A., Bogaard H.J. Risk factors for hemoptysis in idiopathic and hereditary pulmonary arterial hypertension. PLoS ONE. 2013;8:e78132. doi: 10.1371/journal.pone.0078132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rali P., Gandhi V., Tariq C. Massive Hemoptysis. Crit. Care Nurs. Q. 2016;39:139–147. doi: 10.1097/CNQ.0000000000000107. [DOI] [PubMed] [Google Scholar]
- 86.Kim T.H., Koo H.J., Lim C.-M., Hong S.-B., Huh J.W., Jo K.W., Shim T.S., Kim W.S., Koh Y. Risk factors of severe hemoptysis in patients with fungus ball. J. Thorac Dis. 2019;11:4249–4257. doi: 10.21037/jtd.2019.09.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data are created.