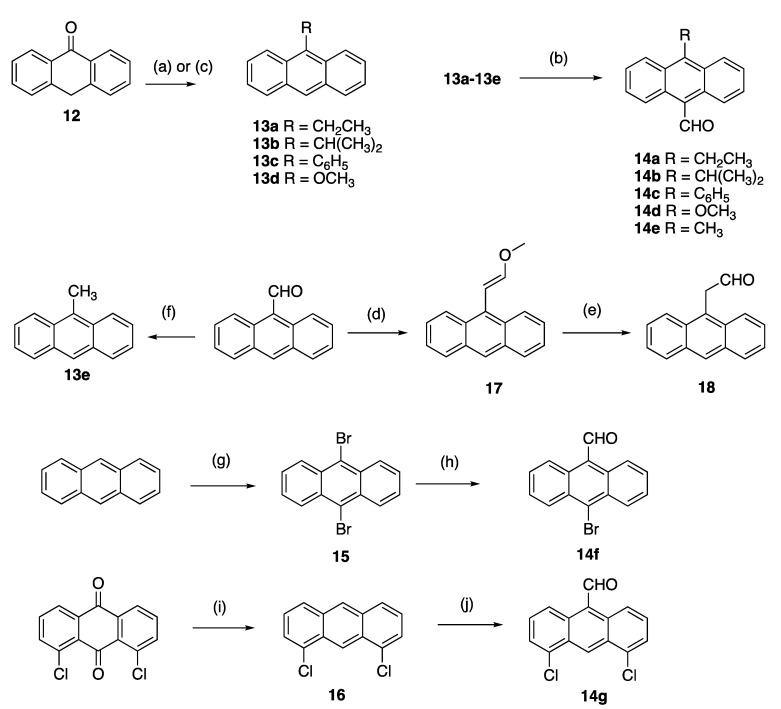
Scheme 2.
Synthesis of substituted 9-anthraldehydes. Scheme reagents and conditions: (a) (step 1) for compounds 13a–c: RMgBr, toluene, reflux, 3 h; (step 2) HCl (20%), ice, (76–84%); (b) N-methylformanilide, POCl3, 100 °C, 1.5 h, (60–80%); (c) for compound 13d: triethylorthoformate, H2SO4, benzene, methanol, reflux, 72 h (76%); (d) CH3OCH2P(Ph3)Cl, THF, KOtBu, 0–25 °C, 2 h, (60%); (e) NaI, TMSCl, CH3CN, 10 min, (83%); (f) NH2NH2, KOH, diethylene glycol 200 °C, pressure tube, 1 h, (84%); (g) bromine, DCM, RT, 4 h, (90%); (h) N-Methylformanilide, n-BuLi, 90 °C, 50 min, (72%); (i) (step 1) Zn dust, ammonia, 75 °C, 4 h; (step 2) HCl, isopropanol, 3 h, reflux, (57%); (j) dichloromethyl methyl ether, AlCl3, DCM, 1 h, (30%).