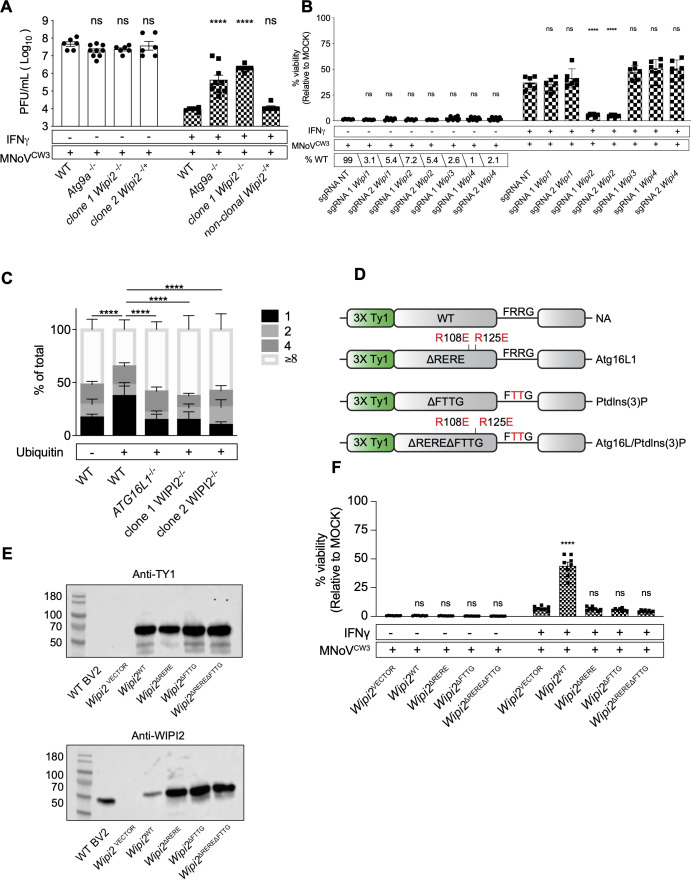
Fig 4.
ATG16L1 and PtdIns(3)P-binding domains of WIPI2B are required for IFNγ-induced inhibition of norovirus cytopathicity in BV-2 cells; WIPI2 is required for IFNγ-induced growth restriction of T. gondii in HeLa cells. (A) Plaque assay of WT, Atg9a−/−, Wipi2−/− clonal, and Wipi2−/+ non-clonal knock-out cells as described in Fig. S1. (B) Viability assay of non-clonal BV-2-Cas9 knock-out cells transduced with non-targeting (NT) sgRNAs or sgRNAs targeting Wipi1, Wipi2, Wipi3, or Wipi4 as described in Fig. S1; percentage of the wild-type allele present in each non-clonal cell pool indicated. (C) WIPI2−/− HeLa cells were treated with IFNγ and infected with T. gondii. Parasites within ubiquitin positive (+) and ubiquitin negative (−) vacuoles were counted by immunofluorescence microscopy. (D) Schematic representation of WIPI2B complementation in clone 1 Wipi2−/− BV-2 cells. (E) Western blot detection of TY1 epitope or endogenous WIPI2 in complemented cells. (F) Viability assay of Wipi2VECTOR, Wipi2WT, Wipi2ΔRERE, Wipi2 ΔRERE, and Wipi2ΔRERE ΔFTTG complemented BV-2 cells as described in Fig. S1. Values in (A), (B), and (E) represent means ± SEM from 2 to 3 independent experiments. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001 were considered statistically significant. ns, not significant. P value was determined by two-way ANOVA with Dunnett’s multiple comparison test; for T. gondii assay, P value was determined by two-way ANOVA with Tukey’s multiple comparison test.