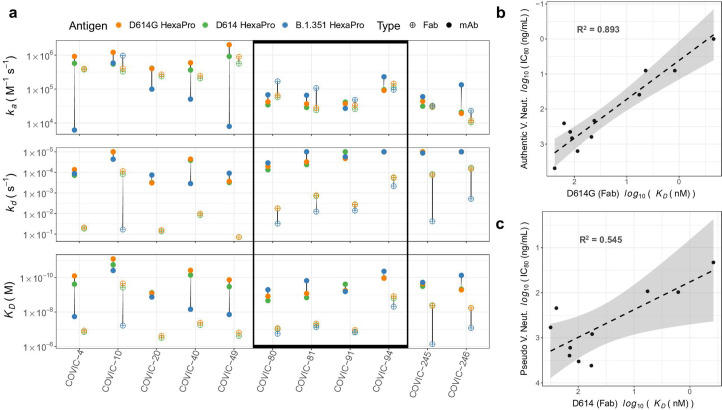
Fig 6.
Fab fragments of CoVIC constructs targeting various binding sites can retain strong affinity to B.1.351. (a) The association rate constant (ka), dissociation rate constant (kd) and affinity values (KD) for each CoVIC construct as a mAb or Fab binding to D614, B.1.351, and D614G HexaPro. Filled and open circles correspond to values for intact IgG and Fab fragments, respectively. No value is displayed for Fab fragments that showed no detectable binding to B.1.351. Constructs showing comparable binding to all three HexaPro proteins as Fabs are indicated within the rectangle. (b) The correlation between Fab binding affinity to D614G HexaPro and the IC80 value for neutralization of authentic D614G virus is shown. (c) The correlation between Fab binding affinity to D614 HexaPro and the IC80 value for pseudovirus neutralization bearing D614 is shown. (b, c) Neutralization data were obtained from CoVIC-DB (https://CoVICdb.lji.org/). R2 values are from linear regression.