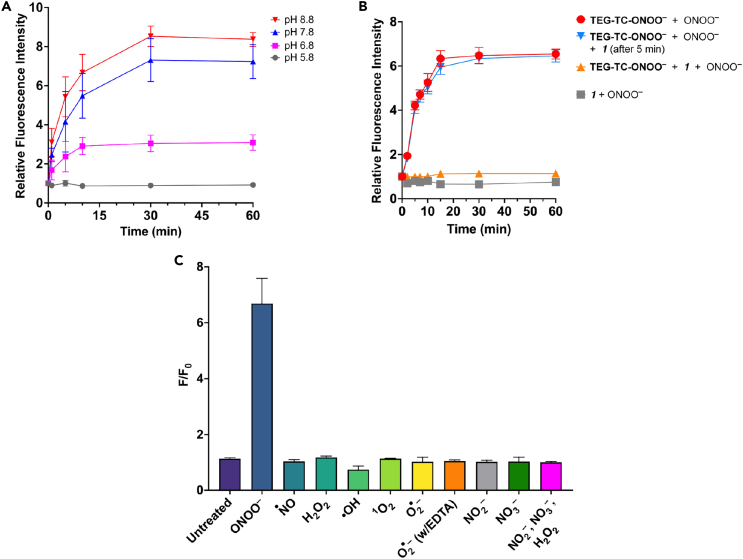
Figure 2.
Peroxynitrite-specific reactivity and redox selectivity of TEG-TC-ONOO–
(A) The time-dependent change in relative fluorescence intensity at 405/475 nm excitation/emission for TEG-TC-ONOO– (1 μM) treated with ONOO− (600 μM) at various pHs: 5.8, 6.8, 7.8, and 8.8. Buffer: 50 mM Tris (pH 6.8, 7.8, or 8.8) or MES (pH 5.8).
(B) The time-dependent change in relative fluorescence intensity at 405/475 nm excitation/emission for TEG-TC-ONOO– and/or 1,1,1-trifluoro-4-(4-hydroxyphenyl)butan-2-one (the ketone 1), treated with ONOO−. TEG-TC-ONOO– (1 μM), 1 (600 μM), and ONOO− (600 μM). The solutions were prepared using Tris 7.5 (50 mM) buffer.
(C) Selectivity of TEG-TC-ONOO– against RONS. TEG-TC-ONOO– (1 μM) was dissolved in Tris buffer (50 mM, pH 7.5) and subjected to RONS with an estimated initial concentration of 600 μM. After the addition of ONOO− (600 μM) into Tris buffer (50 mM), pH increased to 8.7. EDTA (600 μM) was added to chelate a transition metal (e.g., copper) that leads to superoxide dismutation. Data represent F/F0 measurements of samples incubated for 60 min. F and F0 are defined as the fluorescence with and without the redox agent. (a–c) Ex/Em: 405/475 nm. Error bars represent standard deviation, n = 3.