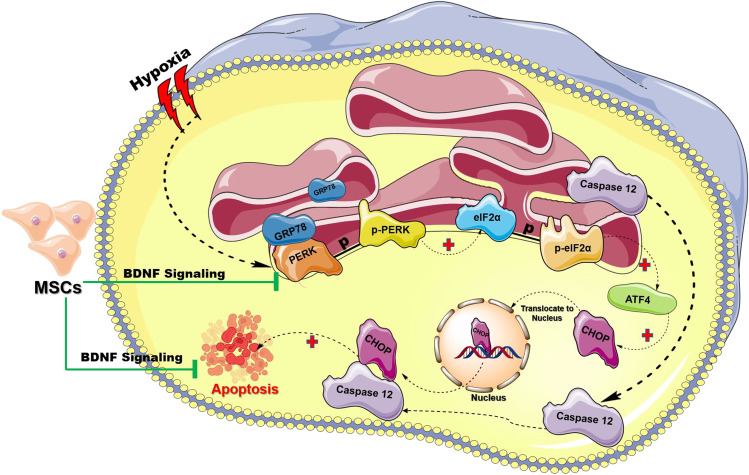
Fig. 7.
Schematic diagrammatic representation of the proposed hypothesis. Hypoxia induces misfolding of protein due to the lack of oxygen which is required for the formation of disulfide linkages and ultimately leads to endoplasmic reticulum (ER) stress. Under basal conditions, GRP78 binds to PERK and prevents its activation. However, in response to ER stress during hypoxia, GRP78 dissociates from PERK, allowing phosphorylation of PERK activation. Activated PERK further phosphorylates eIF2α resulting in a global inhibition of protein synthesis that allows the activation of ATF4, a transcription factor which then induces the expression of target genes CHOP. Once CHOP gets activated, it translocates to the nucleus and regulates the expression of genes involved in cell death (black dotted arrow). Caspase 12 is an ER-localized caspase that has been postulated to play a pivotal role in the ER stress-induced apoptosis pathway (black dotted arrow). Following hypoxia caspase 12 dissociates from ER membrane and translocate to cytosol further cleaves pro-caspase 9 and in turn activate caspase 3, thereby promoting apoptosis. We hypothesize that MSCs-mediated BDNF signaling limits the activation of ER stress and eventually preventing apoptosis to render neuroprotection (green line). (Image adapted from Servier Medical Art by Servier which is licensed under a Creative Commons Attribution 3.0 Unported License; https://smart.servier.com/ and https://biorender.com/)