Abstract
Little is known about the long-term durability of the induced immune response in subjects with obesity, particularly in those with an abdominal distribution of adipose tissue. We evaluated SARS-CoV-2-specific antibody responses after BNT162b2 vaccine booster dose, comparing individuals with and without abdominal obesity (AO), discerning between individuals previously infected or not. IgG-TrimericS were measured in 511 subjects at baseline, on the 21st day after vaccine dose 1, and at 1, 3, 6, and 9 months from dose 2, and at 1 and 3 months following the booster dose. To detect SARS-CoV-2 infection, nucleocapsid antibodies were measured at baseline and at the end of the study. Multivariable linear regression evaluated the three-month difference in the absolute variation in IgG-TrimericS levels from booster dose, showing AO and SARS-CoV-2 infection status interactions (p = 0.016). Regardless of possible confounding factors and IgG-TrimericS levels at the booster dose, AO is associated with a higher absolute change in IgG-TrimericS in prior infected individuals (p = 0.0125). In the same regression model, no interaction is highlighted using BMI (p = 0.418). The robust response in the development of antibodies after booster dose, observed in people with AO and previous infection, may support the recommendations to administer a booster dose in this population group.
Keywords: abdominal obesity, obesity, BMI, BNT162b2 mRNA vaccine, antibody response, IgG-TrimericS, booster dose, COVID-19
1. Introduction
People with chronic medical conditions have an increased risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and of developing major complications from coronavirus disease 2019 (COVID-19) [1]. Genetic and environmental host factors, including age, biological sex, comorbidities, and adipose tissue distribution converge to influence innate and adaptive immune responses to vaccines [2]. Individuals with obesity have an increased risk of contracting and developing a more severe case of COVID-19, especially those with a predominant accumulation of visceral adipose tissue (VAT), making these individuals an at-risk population [3,4,5]. Excessive VAT accumulation leads to low-grade systemic inflammation and predisposes individuals to an increased susceptibility to infection, increased morbidity and mortality, and reduced development of antibodies to vaccines due to a reduced immune response [6].
Moreover, abdominal adiposity is characterized by increased visceral and ectopic fat deposition, adipocyte dysfunction, inflammatory and adipokine dysregulation and insulin resistance as emerging risk factor for type 2 diabetes mellitus, hypertension, cardiovascular diseases, and fatty liver, conditions that could lead to more severe forms of infections, especially SARS-CoV-2 [5,7].
Approved messenger RNA (mRNA) vaccines against SARS-CoV-2 are highly effective at reducing infection and morbidity in the general population and have been highly recommended for individuals affected by obesity [8]. It is hypothesised that the chronic inflammatory state, immune dysregulation, and any related comorbidities, characteristic of subjects with obesity, particularly at the visceral level, predispose to a low immunological response to various vaccinations [5,9,10]. To date, the efficacy of vaccines does not seem to differ significantly in individuals affected by obesity or not [8,11]. Therefore, encouragement to receive vaccination is strongly advisable for people suffering from obesity [8]. However, the impact of obesity and abdominal obesity (AO) on the durability of mRNA vaccine-specific responses remains an open question [12,13].
We and others have previously reported a weaker immune response after two doses of BNT162b2 (Pfizer–BioNTech) and a greater drop in antibody levels at three months after dose 2 in infection-naïve subjects with AO compared with those without [14,15,16]. Therefore, the observed reduction in antibody levels over time after vaccination and the increase in positive cases has led to the need for additional booster vaccination [14,17,18,19,20].
The long-term duration of the immune response induced by the vaccination cycle and booster dose is still little understood, especially in patients with AO. In addition, uncertainties remain about the effect of vaccination on development of antibodies in individuals with previous infection [21,22,23,24,25].
To this end, we assessed SARS-CoV-2-specific antibody responses after the booster dose of the BNT162b2 mRNA vaccine in a cohort of health care workers. We compared the antibody response of individuals with or without AO, discerning their infection status (infection-naïve individuals and individuals infected pre or post vaccination cycle).
2. Material and Methods
2.1. Study Design and Population
In our study, the population was recruited in the prospective observational cohort study VARCO-19, that began in January 2021 and ended in March 2022 at IRCCS Policlinico San Donato, Italy. All participants gave written informed consent, and the protocol received approval from the IRCCS Lazzaro Spallanzani Ethics Committee (protocol code 48/2021/spall/PU/403-2021).
We collected blood samples from health care workers who received up to three doses of the BNT162b2 mRNA vaccine. The vaccination itself was not part of the study. A description of the enrolment process and inclusion criteria has previously been reported [14].
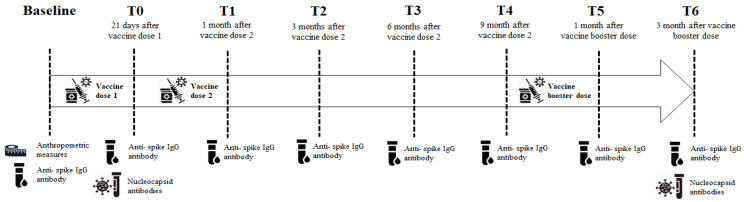
The study timeline is shown in Figure 1.
Figure 1.
Study timeline. This figure shows all the events of the study.
2.2. Serological Testing
Serological testing was performed as previously described [14]. The Binding Antibody Unit (BAU)/mL was used as the unit of measurement for serological tests because it represents the unit of measurement imposed by the World Health Organisation (WHO) to standardise the values of antibodies that are measured with different methods in different laboratories around the world (≥33.8 BAU/mL is the minimum threshold of protection). The conversion factor from Arbitrary Unit AU/ML to BAU/mL is 2.6. Levels of antibodies were tested at eight time points: at baseline, 21 days following vaccine dose 1, and 1 (within 30–40 days), 3 (within 90–100 days), 6 (within 180–200 days), and 9 months (within 270–280 days) from vaccine dose 2. In addition, antibody levels were measured at 1 (within 30–40 days) and 3 months (within 90–100 days) from the booster dose. When the antibody determination was above the test range upper limit, a 1:20 dilution was performed using the specific buffer LIAISON® TrimericS IgG Diluent Accessory (DiaSorin, Saluggia, Italy). Sporadic titres of >40,000 BAU/mL were obtained because of the limitation of the linear range at a dilution of 1:20. Since this was infrequent, no further dilutions were performed, and these samples were given a titre of 40,000 BAU/mL. At the beginning of the study, a qualitative evaluation of anti-nucleocapsid IgG (anti-N IgG) was performed to check whether SARS-CoV-2 infections had occurred prior to vaccination. To assess whether a participant had been infected with SARS-CoV-2 during the VARCO-19 study, we determined the anti-N IgG titre again three months after the booster dose.
2.3. Anthropometric Measures
Anthropometric measurements were evaluated at baseline. We determined waist circumference using a flexible tape measure by setting it midway between the iliac crest and the lower rib, to the nearest 1.0 cm. AO was defined as a waist circumference ≥ 102 cm in men and ≥88 cm in women [26]. Body weight was measured to the nearest 0.1 kg using a beam scale, and height was measured to the nearest 0.1 cm using a stadiometer. Body mass index (BMI) was calculated as the weight (km) divided by height (m) squared.
2.4. Statistical Analyses
Antibody levels were expressed as the geometric mean (±standard deviation, SD). Subjects were classified according to AO and BMI classes with or without a previous diagnosis of SARS-CoV-2 infection (no prior infection, infection diagnosis before vaccine or infection diagnosis after vaccine). Comparison of continuous values between groups was performed using the non-parametric Kruskal–Wallis test. Multivariable linear regression was used to account for possible confounding and to assess absolute variation: difference at three months of titre levels from booster dose in individuals with AO and without (or BMI-classes). The model was also adjusted for antibody levels from the booster dose, gender, age, smoking, hypertension, and previous diagnosis of SARS-CoV-2 infection and the interaction between previous infection and AO (or BMI classes). Least-squares (LS) means (±standard error, SE) were reported. The null hypothesis was rejected at p < 0.05. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
3. Results
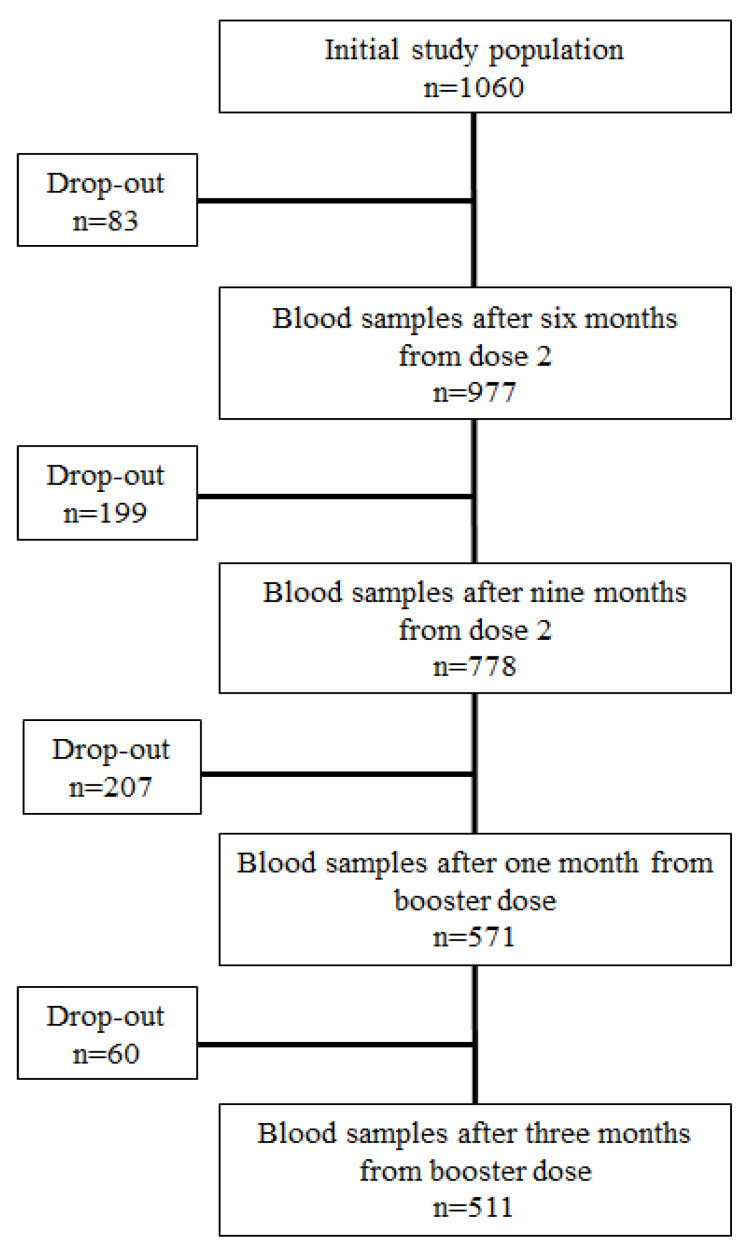
The initial study population consisted of 1060 employees of the IRCCS Policlinico San Donato, who received a BNT162b mRNA vaccine and from whom at least one blood sample was collected for antibody testing. The subjects enrolled in the VARCO-19 study were 41.4 ± 12.9 years old, 93% were Caucasian, and 62% were female: 1060 subjects who underwent vaccination (240 previously infected) provided samples 21 days following vaccine dose 1, after 1 month (within 30–40 days), and after 3 months (within 90–100 days) from dose 2; 977 (218 previously infected) provided samples at 6 months (within 180–200 days) after dose 2; 778 (177 with prior infection) provided samples at 9 months (within 270–280 days) after dose 2; 571 (126 with prior infection) provided samples at 1 month (within 30–40 days) after booster dose; 511 (109 prior infected individuals) provided samples after 3 months (within 90–100 days) following the booster dose (Figure 2).
Figure 2.
Study population flow diagram.
In total, 511 individuals provided blood samples at all time points and were included in this analysis. Baseline characteristics of the patients are reported in Table 1.
Table 1.
Demographic and clinical traits at baseline of the entire study population stratified by the presence or absence of abdominal obesity and infection status.
| With Abdominal Obesity (n = 149) | Without Abdominal Obesity (n = 362) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n = 511) |
Without Prior SARS-CoV-2 Infection (n = 76) | With Prior SARS-CoV-2 Infection (n = 34) |
SARS-CoV-2 Infection after Vaccine (n = 39) |
p-Value | Without Prior SARS-CoV-2 Infection (n = 186) | With Prior SARS-CoV-2 Infection (n = 75) | SARS-CoV-2 Infection after Vaccine (n = 101) |
p-Value | |
| Age, years | 44.03 ± 11.88 | 52.02 ± 10.64 | 47.26 ± 9.18 | 48.26 ± 8.28 | 0.0286 | 42.28 ± 12.02 | 41.73 ± 11.20 | 40.15 ± 11.70 | 0.3386 |
| Ethnicity | |||||||||
| Caucasian | 492 (96.28) | 72 (94.74) | 31 (91.18) | 36 (92.31) | 181 (97.31) | 71 (94.67) | 101 (100.00) | ||
| Latin-American | 13 (2.54) | 3 (3.95) | 3 (8.82) | 3 (7.69) | 0.7117 * | 2 (1.08) | 2 (2.67) | 0 (0.00) | 0.2074 * |
| African | 2 (0.39) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (1.08) | 0 (0.00) | 0 (0.00) | ||
| Arabic | 4 (0.78) | 1 (1.32) | 0 (0.00) | 0 (0.00) | 1 (0.54) | 2 (2.67) | 0 (0.00) | ||
| Gender | |||||||||
| Male | 166 (32.49) | 30 (39.47) | 8 (23.53) | 14 (35.90) | 0.2656 | 56 (30.11) | 28 (37.33) | 30 (29.70) | 0.4271 |
| Female | 345 (67.51) | 46 (60.53) | 26 (76.47) | 25 (64.10) | 130 (69.89) | 47 (62.67) | 71 (70.30) | ||
| Smoking status | |||||||||
| Smoker | 98 (19.18) | 11 (14.47) | 4 (11.76) | 10 (25.64) | 0.2128 | 36 (19.35) | 11 (16.67) | 26 (25.74) | 0.1794 |
| Non-smoker | 413 (80.82) | 65 (85.53) | 30 (88.24) | 29 (74.36) | 150 (80.65) | 64 (85.33) | 75 (74.26) | ||
| Comorbidities | |||||||||
| Hypertension | 59 (11.55) | 25 (32.89) | 7 (20.59) | 6 (15.38) | 0.0944 | 12 (6.45) | 3 (4.00) | 6 (5.94) | 0.7435 |
| Diabetes mellitus | 4 (0.78) | 0 (0.00) | 2 (5.88) | 0 (0.00) | 0.0509 * | 0 (0.00) | 1 (1.33) | 1 (0.99) | 0.2357 * |
| Cardiovascular diseases | 17 (3.33) | 6 (7.89) | 0 (0.00) | 1 (2.56) | 0.2318 * | 7 (3.76) | 1 (1.33) | 2 (1.98) | 0.5268 * |
| Dyslipidaemia | 32 (6.26) | 9 (11.84) | 4 (11.76) | 5 (12.82) | 1.000 * | 7 (3.76) | 3 (4.00) | 4 (3.96) | 1.000 * |
| Cancer | 3 (0.59) | 1 (1.32) | 0 (0.00) | 1 (2.56) | 1.000 * | 1 (0.54) | 0 (0.00) | 0 (0.00) | 1.000 * |
| Anthropometric measurements | |||||||||
| Weight, kg | 70.54 ± 15.12 | 84.08 ± 13.74 | 88.28 ± 16.96 | 80.86 ± 14.45 | 0.1026 | 64.38 ± 11.15 | 65.40 ± 12.29 | 65.53 ± 10.23 | 0.6481 |
| Height, cm | 167.63 ± 8.78 | 168.47 ± 10.54 | 166.14 ± 9.01 | 167.21 ± 8.61 | 0.4903 | 166.91 ± 8.39 | 168·21 ± 8.65 | 168.56 ± 8.10 | 0.2252 |
| Waist, cm | 85.78 ± 13.51 | 100.69 ± 8.76 | 103.26 ± 11.23 | 99.79 ± 9.47 | 0.2745 | 79.61 ± 9.20 | 76.63 ± 9.23 | 79.19 ± 9.22 | 0.9254 |
| Waist male, cm | 94.63 ± 11.85 | 107.25 ± 5.64 | 112.88 ± 9.79 | 107.93 ± 6.56 | 0.1104 | 89.26 ± 7.47 | 87.38 ± 7.93 | 87.75 ± 7.91 | 0.4994 |
| Waist female, cm | 81.52 ± 12.13 | 96.41 ± 7.74 | 100.31 ± 10.05 | 95.24 ± 7.64 | 0.0751 | 75.46 ± 6.31 | 75.02 ± 6.49 | 75.58 ± 7.13 | 0.8975 |
| WHtR | 0.51 ± 0.08 | 0.60 ± 0.05 | 0.62 ± 0.06 | 0.60 ± 0.05 | 0.0589 | 0.48 ± 0.05 | 0.47 ± 0.05 | 0.47 ± 0.05 | 0.5170 |
| BMI, kg/m2 | 25.00 ± 4.45 | 29.53 ± 3.28 | 31.79 ± 4.39 | 28.81 ± 3.76 | 0.00019 | 23.02 ± 2.91 | 22.96 ± 2.87 | 23.02 ± 2.89 | 0.9869 |
| BMI classes | |||||||||
| Underweight | 20 (3.91) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 12 (6.45) | 2 (2.67) | 6 (5.94) | ||
| Normal weight | 265 (51.86) | 7 (9.21) | 1 (2.94) | 7 (17.95) | 0.0951 * | 129 (69.35) | 51 (68.00) | 70 (69.31) | 0.8376 * |
| Overweight | 156 (30.53) | 34 (44.74) | 13 (38.24) | 20 (51.28) | 43 (23.12) | 22 (29.33) | 24 (23.76) | ||
| Obesity | 70 (13.70) | 35 (46.05) | 20(58.82) | 12 (30.77) | 2 (1.08) | 0 (0.00) | 1 (0.99) | ||
* Fisher test. Abdominal obesity (waist circumference ≥102 cm for men, ≥88 cm for women); no abdominal obesity (waist circumference < 102 cm for men, <88 cm for women); waist-to-height ratio (WHtR); Body Mass Index (BMI); underweight (BMI < 18.5 kg/m2); normal weight (BMI from 18.5 to <25 kg/m2); overweight (BMI from 25 to <30 kg/m2); obesity (BMI ≥ 30 kg/m2). Antibody levels are expressed as BAU/mL (BAU, Binding Antibody Units) and are presented as the geometric mean [95% confidence interval]. Data are n (%), mean (SD).
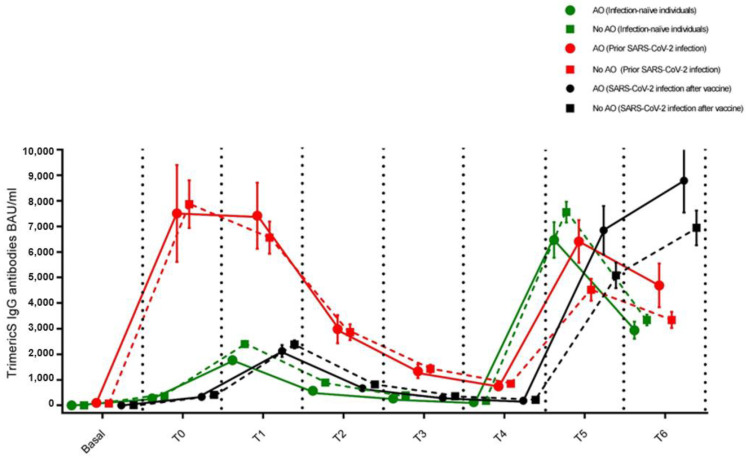
We divided our sample according to two parameters: the presence of AO and the infection status. According to the waist circumference cut-off, 149 subjects (29.1%) were affected by AO and 362 (70.9%) exhibited normal adipose tissue distribution. According to infection status, subjects were divided into three categories: (1) those who had never been infected with SARS-CoV-2 (infection-naïve individuals, n = 262, 51.3%); (2) those who had developed the infection before the vaccine cycle (prior infected individuals, n = 109, 21.3%); and (3) those who developed the infection during the vaccine cycle (post infected individuals, n = 140, 27.4%). Figure 3 shows the IgG-TrimericS antibody response to mRNA SARS-CoV-2 vaccination in individuals with or without AO according to infection status at all time points.
Figure 3.
IgG-TrimericS antibody response to mRNA SARS-CoV-2 vaccination in subjects affected or not by abdominal obesity according to infection status. Infection-naïve individuals (green), individuals with prior infection (red), and individuals infected with SARS-CoV-2 after booster dose (black). Abdominal obesity (waist circumference ≥ 102 cm for men, ≥88 cm for women); no abdominal obesity (waist circumference < 102 cm for men, <88 cm for women).
3.1. Individuals Who Had Never Been Infected with SARS-CoV-2 (Infection-Naïve Individuals)
Among infection-naïve individuals, n = 262, 76 (29%) with and 186 (71%) without AO, between the third and ninth month after vaccine dose 2, there was a decrease in IgG-TrimericS levels in both subjects with AO and those without AO (0.22-fold [95% CI: 0.16–0.29] vs. 0.20-fold [95% CI: 0.17–0.23], respectively, Table 2, Figure 3). At one and three months after the vaccine booster dose, in subjects with AO, IgG-TrimericS levels were found to be lower than in individuals not suffering from AO without reaching statistical significance. An antibody peak was shown at one month after the vaccine booster dose (geometric mean BAU/mL ± standard deviation, 6470.55 ± 695.82 BAU/mL in individuals with AO vs. 7561.64 ± 406.80 BAU/mL in individuals without AO, p = 0.173), and a decline at the third month (2943.17 ± 335.19 BAU/mL in individuals with AO vs. 3346.92 ± 208.06 BAU/mL in individuals without AO, p = 0.413, Table 2, Figure 3).
Table 2.
IgG-TrimericS antibody levels of subjects who provided a blood sample at all time points stratified by the presence or absence of abdominal obesity and SARS-CoV-2 infection status.
| With Abdominal Obesity (n = 149) | Without Abdominal Obesity (n = 362) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Antibody Levels | Total (n = 511) |
Without Prior SARS-CoV-2 Infection (n = 76) | With SARS-CoV-2 Infection before Vaccine (n = 34) |
SARS-CoV-2 Infection after Vaccine (n = 39) |
p-Value | Without SARS-CoV-2 Infection (n = 186) | With SARS-CoV-2 Infection before Vaccine (n = 75) | SARS-CoV-2 Infection after Vaccine (n = 101) |
p-Value |
| Baseline | 9.67 ± 1.40 n = 90 |
4.81 n = 11 |
98.93 ± 74.44 n = 6 |
4.81 n = 8 |
<0.0001 *° |
5.59 ± 0.58 n = 32 |
71.81 ± 24.26 n = 13 |
6.11 ± 1.19 n = 20 |
<0.0001 *° |
| 21 days after dose 1 |
680.84 ± 49.58 | 274.16 ± 36.40 | 7511.83 ± 1895.77 | 333.58 ± 61.94 | <0.0001 *° |
362.54 ± 26.99 | 7872.56 ± 931.04 | 410.72 ± 32.39 | <0.0001 *° |
| 1 month after dose 2 |
2837.32 ± 110.30 | 1773.18 ± 155.93 | 7420.51 ± 1296.00 | 2130.24 ± 231.39 | <0.0001 *° |
2399.17 ± 123.48 | 6562.31 ± 631.09 | 2386.67 ± 156.08 | <0.0001 *° |
| 3 months after dose 2 |
1035.22 ± 43.80 | 575.90 ± 53.28 | 2985.81 ± 550.26 | 672.12 ± 68.08 | <0.0001 *° |
892.48 ± 44.54 | 2868.23 ± 308.43 | 820.87 ± 60.84 | <0.0001 *° |
| 6 months after dose 2 |
463.96 ± 21.32 | 267.78 ± 14.63 | 1335.15 ± 267.46 | 296.44 ± 30.03 | <0.0001 *° |
391.60 ± 22.71 | 1431.65 ± 155.39 | 358.84 ± 29.21 | <0.0001 *° |
| 9 months after dose 2 |
243.18 ± 11.93 | 126.78 ± 14.62 | 746.25 ± 131.40 | 183.41 ± 32.79 | <0.0001 *° |
176.38 ± 10.74 | 847.73 ± 92.92 | 216.88 ± 17.25 | <0.0001 *° |
| 1 month after booster dose |
6218.25 ± 237.47 | 6470.55 ± 695.82 | 6413.54 ± 832.40 | 6850.16 ± 947.21 | 0.8892 | 7561.64 ± 406.80 | 4521.62 ± 429.76 | 5084.36 ± 500.43 | <0.0001 #* |
| 3 months after booster dose |
4175.81 ± 181.19 | 2943.17 ± 335.19 | 4692.80 ± 854.60 | 8791.71 ± 1246.30 | <0.0001 #° |
3346.92 ± 208.06 | 3345.35 ± 314.00 | 6944.18 ± 682.39 | <0.0001 #° |
Abdominal obesity (waist circumference ≥ 102 cm for men, ≥88 cm for women); no abdominal obesity (waist circumference < 102 cm for men, <88 cm for women). * (SARS-CoV-2 infection before vaccine vs. no prior SARS-CoV-2 infection) # (SARS-CoV-2 infection after vaccine vs. no prior SARS-CoV-2 infection) ° (SARS-CoV-2 infection before vaccine vs. SARS-CoV-2 infection after vaccine).
3.2. Individuals Who Had Developed the Infection before the Vaccine Cycle (Prior Infected Individuals)
Among prior infected individuals, n = 109, 34 (31.2%) with and 75 (68.8%) without AO, between month three and month nine following the second dose of vaccine, in both subjects with AO and those without AO, the drop in IgG-TrimericS levels was significant (0.25-fold [95% CI: 0.15–0.42] vs. 0.20-fold [95% CI: 0.30–0.33], respectively, Table 2, Figure 3).
One month following the administration of the vaccine booster dose, a higher peak in IgG-TrimericS levels was reached in subjects affected by AO compared with those not affected by AO (6470.55 ± 695.82 BAU/mL vs. 4521.62 ± 429.76 BAU/mL, p = 0.0521). In the same two groups, between the first and third month after the booster dose, a similar drop in IgG-TrimericS levels was recorded (0.73-fold [95% CI: 0.48–1.14] vs. 0.74-fold, [95% CI: 0.57–0.96], p = 0.2352, Table 2, Figure 3).
3.3. Individuals Who Developed the Infection during the Vaccine Cycle
One hundred and forty individuals, 39 (27.9%) with and 101 (72.1%) without AO, became positive for SARS-CoV-2 anti-nucleocapsid IgG antibodies testing at the end of the observation period, indicating that they had contracted COVID-19 during the vaccine cycle. The most observed symptoms were mild, ranging from asymptomatic to mild fever. Among individuals who presented symptoms (n = 54), there were 38 (70.3%) individuals with AO and 16 (29.7%) individuals without AO. Between the third and ninth month after vaccine dose 2, there was a decrease in IgG-TrimericS levels in both subjects with AO and those without AO (0.27-fold [95% CI: 0.18–0.41] vs. 0.26-fold [95% CI: 0.21–0.3], respectively), similarly to infection-naïve individuals (Table 2, Figure 3). One month after the vaccine booster dose, individuals affected by AO exhibited comparable levels of IgG-TrimericS to those not affected by AO (6850.16 ± 947.21 BAU/mL vs. 5084.36 ± 500.43 BAU/mL, p = 0.119). Moreover, during the period from the first to the third month following the booster dose of the vaccine, individuals with AO achieved a higher peak of IgG-TrimericS levels compared with those not suffering from AO, even without statistical significance (8791.71 ± 1246.30 BAU/mL vs. 6944.18 ± 682.39 BAU/mL, p = 0.242, Table 2, Figure 3).
3.4. Multivariable Linear Regression Analysis
Multivariable linear regression, used to assess the difference at three months in the absolute change in IgG-TrimericS levels from the booster dose, showed evidence of an interaction between AO and SARS-CoV-2 infection (p = 0.016; Table 3). AO, in particular, was associated with a greater absolute variation in IgG-TrimericS in previously infected subjects regardless of sex, age, hypertension, or smoking, and IgG-TrimericS levels at the booster dose (LS means 8432.09 ± 1191.67 vs. 5091.93 ± 918.62, p = 0.0125). In the same regression model, no discernible interaction was identified when utilizing BMI classes (p = 0.418).
Table 3.
Differences in absolute changes in IgG-TrimericS antibody levels from the booster dose up to three months assessed by univariate and multivariate linear regression (p-value).
| p-Value | |||
|---|---|---|---|
| Univariate | Multivariable According To Abdominal Obesity | Multivariable According to BMI Class | |
| Sex | 0.7120 | 0.5955 | 0.5656 |
| Age | 0.3000 | 0.4485 | 0.5412 |
| IgG-TrimetricS antibody level at booster dose | 0.0675 | 0.0855 | 0.0526 |
| Prior SARS-CoV-2-infection | <0.0001 | <0.0001 | <0.0001 |
| Abdominal obesity | 0.1053 | 0.1092 | - |
| Interaction with prior SARS-CoV-2-infection * Abdominal obesity | 0.0163 | - | |
| BMI classes | 0.0803 | - | 0.0821 |
| Interaction with prior SARS-CoV-2-infection * BMI classes | - | 0.4176 | |
| Smoking status | 0.0925 | 0.3785 | 0.2968 |
| Hypertension | 0.0062 | 0.0020 | 0.0063 |
| Diabetes mellitus | 0.5878 | ||
| Cardiovascular diseases | 0.7262 | ||
| Dyslipidemia | 0.5087 | ||
| Cancer | 0.3900 | ||
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); abdominal obesity (waist circumference ≥ 102 cm for men, ≥88 cm for women); Body Mass Index (BMI).
4. Discussion
In this longitudinal, observational study, VARCO-19, we presented data on antibody levels in a cohort of health care workers up to twelve months after a vaccination cycle with BNT162b2 mRNA and at month three following a booster dose.
In line with other studies, our findings showed a peak in antibody levels one month after the second vaccine dose, succeeded by a gradual decline until the administration of the booster dose. This trend was observed in individuals with or without AO, regardless of prior SARS-CoV-2 infection, whether before or during the vaccination cycle.
Previous studies have shown that humoral responses following dose 2 of the COVID-19 vaccine decrease in all population groups approximately six months after the second dose of a vaccine [18,27]. The decline in humoral responses does not depend on the type of COVID-19 vaccine administered, but on host factors [14,15,28,29,30,31]. Overall, it has already been shown that the humoral immune response to vaccination differs significantly between individuals previously infected with SARS-CoV-2 and naïve individuals [32].
We found that in all infection-naïve individuals, IgG-TrimericS concentrations decreased steadily after nine months from the second dose and after three months from the booster dose of a BNT162b2 m-RNA vaccine. Similarly to our previous study [14] and in according with other studies [16,33], infection-naïve individuals with AO, at one and three months after vaccine booster dose, have lower antibody levels than individuals without AO, suggesting that the duration of vaccine-induced immunity may be reduced in people with obesity. Watanabe et al. previously demonstrated an association between central obesity and a reduced adaptive response to the BNT162b2 m-RNA vaccine [29]. Furthermore, weight loss and/or improved metabolic health appears to reverse the effect. Adipose tissue, in addition to serving as a lipid store and energy source, is an endocrine organ that secretes fatty acids, metabolites, and adipokines, which have a crucial function in inflammation and the immune response, negatively influenced by the production of proinflammatory adipokines and cytokines [5,34,35,36,37]. In subjects affected by general obesity, and especially by AO, chronic inflammation, developed due to dysfunctional adipose tissue, negatively affects T-cell function, macrophage migration, and antibody response [38,39,40]. This is due to an overaccumulation of adipose tissue, particularly at the abdominal level, which can cause the secretion of adipokines and pro-inflammatory cytokines, which negatively impact the immune response [34].
Thus, immune dysfunction may increase the risk of developing SARS-CoV-2 infection and may decrease the response to the vaccine in naïve individuals with severe obesity [39,41].
We showed that, one month after vaccine booster dose, prior infected individuals with AO had higher concentrations of IgG-TrimericS than individuals without AO. This could be due to a more severe infection that is more frequent in patients with obesity [42,43].
Previous research, in fact, has shown that the level of antibodies to COVID-19 is associated with disease severity and that obesity correlates with a higher risk of contracting a more severe form of COVID-19 [44,45]. Subjects affected by severe obesity who survive COVID-19 generate robust and long-lasting SARS-CoV-2-specific T-cell immunity following severe infection; this is more evident in patients with obesity [46,47]. Furthermore, Muena et al. showed that immunization with CoronaVac or BNT162b2 vaccines, administered up to 13.3 months after the onset of COVID-19 symptoms, can significantly improve long-lasting neutralizing antibody responses induced by natural infection, suggesting that the infection induces a robust immune system response [48]. Hybrid immunity, resulting from the combination of prior SARS-CoV-2 infection and vaccination, appeared to confer enhanced protection against SARS-CoV-2 infections [49,50]. A booster dose administered after natural COVID-19 infection appears to provide a more consistent humoral immune response in terms of magnitude and quality than vaccination in infection-naïve individuals, fully consistent with clinical epidemiological observations [32]. This could explain the hyper-antibody response to the vaccine in this population, as reflected in our results.
Our data show that 27.4% of our population contracted COVID-19 despite having undergone the vaccination cycle and having high antibody levels. However, none of these subjects showed severe symptoms, but only mild manifestations such as fatigue, cold, sore throat, low-grade fever, and joint pain, suggesting that hybrid immunity could protect from severe COVID-19 illness. These results are consistent with a previous study showing that hybrid immunity and booster vaccination were associated with a lower risk of SARS-CoV-2 infection and fewer symptoms [51]. When ignoring the booster, any additional protection of two-dose vaccination in infection-naïve individuals was no longer observed [51]. Therefore, booster vaccination may reduce the risk of symptomatic SARS-CoV-2 infection in infection-naïve individuals, although this benefit appears to wane over time [51].
One of the limitations of our study includes the enrolment of only health care workers, who may not be representative of the general Italian population because they are much more exposed to the risk of SARS-CoV-2 infection. Another limitation of our study is the lack of measurement of virus-specific T cells. Anthropometric measurements were assessed only once. Furthermore, we did not evaluate proinflammatory markers of infection and do not know whether the vaccine BNT162b2 is protective against the Omicron XBB.15.
5. Conclusions
Abdominal obesity is a risk factor for severe COVID-19 complications. Currently, there are no reports of significant differences in COVID-19 vaccine effectiveness between people affected or not by obesity. Our results showed a robust response in the development of antibodies against COVID-19 after a BNT162b2 booster dose in people with AO who have been exposed to the virus. Our findings further support the recommendation that adults with high-risk medical conditions, including obesity, and, in particular AO, be vaccinated with booster doses, even if they have already come into contact with the virus.
Our results are based on a cohort of 511 patients; therefore, it may be interesting in future studies to evaluate antibody development after vaccine booster doses in a larger cohort more representative of the general population.
Acknowledgments
The authors acknowledge all the staff of IRCCS Policlinico San Donato. A special mention for all the nurses of IRCCS Policlinico San Donato for the valued help in blood sample collection. Finally, the authors would like to thank Sara Basilico, Michele Iovane, and Gloria Capitanio for their time and effort placed into this study.
Author Contributions
Conceptualization, A.E.M., C.D., C.B. (Caterina Bertolini), L.M., M.C., G.I., E.N. and M.M.C.R.; methodology, C.D., R.C. and L.V.R.; formal analysis, V.M., S.B., C.B. (Carola Buscemi) and F.A.; acquisition, A.E.M., C.D., C.M., L.V.R., M.B.T., V.S., M.T.C., E.C. and C.R.; resources, A.E.M., L.M., M.M.C.R. and R.C.; data curation, C.D., C.M. and M.B.T.; writing—original draft preparation, A.E.M., C.D., V.M., S.B., C.M., C.B. (Caterina Bertolini), C.B. (Carola Buscemi), V.S., M.T.C., L.M., E.C., F.A., C.R., M.C., G.I., E.N. and M.M.C.R.; writing—review and editing, A.E.M., C.D., C.M., C.B. (Caterina Bertolini), C.B. (Carola Buscemi), M.B.T., V.S., M.T.C., E.C., C.R., M.C., G.I. and E.N.; funding acquisition, A.E.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the IRCCS Lazzaro-Spallanzani (protocol code 48/2021/spall/PU/403–2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This study was partially supported by Ricerca Corrente funding from the Italian Ministry of Health to IRCCS Policlinico San Donato and by the GSD Foundation.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Stefan N., Birkenfeld A.L., Schulze M.B. Global pandemics interconnected—Obesity, impaired metabolic health and COVID-19. Nat. Rev. Endocrinol. 2021;17:135–149. doi: 10.1038/s41574-020-00462-1. [DOI] [PubMed] [Google Scholar]
- 2.Tomalka J.A., Suthar M.S., Deeks S.G., Sekaly R.P. Fighting the SARS-CoV-2 pandemic requires a global approach to understanding the heterogeneity of vaccine responses. Nat. Immunol. 2022;23:360–370. doi: 10.1038/s41590-022-01130-4. [DOI] [PubMed] [Google Scholar]
- 3.Busetto L., Bettini S., Fabris R., Serra R., Dal Pra C., Maffei P., Rossato M., Fioretto P., Vettor R. Obesity and COVID-19, An Italian Snapshot. Obesity. 2020;28:1600–1605. doi: 10.1002/oby.22918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Földi M., Farkas N., Kiss S., Dembrovszky F., Szakács Z., Balaskó M., Erőss B., Hegyi P., Szentesi A. Visceral Adiposity Elevates the Risk of Critical Condition in COVID-19, A Systematic Review and Meta-Analysis. Obesity. 2021;29:521–528. doi: 10.1002/oby.23096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanoudou D., Hill M.A., Belanger M.J., Arao K., Mantzoros C.S. Obesity, metabolic phenotypes and COVID-19. Metabolism. 2022;128:155121. doi: 10.1016/j.metabol.2021.155121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honce R., Schultz-Cherry S. Impact of Obesity on Influenza A Virus Pathogenesis, Immune Response, and Evolution. Front. Immunol. 2019;10:1071. doi: 10.3389/fimmu.2019.01071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Leeuw A.J., Oude Luttikhuis M.A., Wellen A.C., Müller C., Calkhoven C.F. Obesity and its impact on COVID-19. J. Mol. Med. 2021;99:899–915. doi: 10.1007/s00109-021-02072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butsch W.S., Hajduk A., Cardel M.I., Donahoo W.T., Kyle T.K., Stanford F.C., Zeltser L.M., Kotz C.M., Jastreboff A.M. COVID-19 vaccines are effective in people with obesity: A position statement from The Obesity Society. Obesity. 2021;29:1575–1579. doi: 10.1002/oby.23251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tubjaroen C., Prachuapthunyachart S., Potjalongsilp N., Sodsai P., Hirankarn N., Jaru-Ampornpan P., Chongsrisawat V. Immunogenicity of an mRNA-Based COVID-19 Vaccine among Adolescents with Obesity or Liver Transplants. Vaccines. 2022;10:1867. doi: 10.3390/vaccines10111867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasr M.-J.C., Geerling E., Pinto A.K. Impact of Obesity on Vaccination to SARS-CoV-2. Front. Endocrinol. 2022;13:898810. doi: 10.3389/fendo.2022.898810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piernas C., Patone M., Astbury N.M., Gao M., Sheikh A., Khunti K., Shankar-Hari M., Dixon S., Coupland C., Aveyard P., et al. Associations of BMI with COVID-19 vaccine uptake, vaccine effectiveness, and risk of severe COVID-19 outcomes after vaccination in England: A population-based cohort study. Lancet Diabetes Endocrinol. 2022;10:571–580. doi: 10.1016/S2213-8587(22)00158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bates J.T., Farmer A.P., Bierdeman M.A., Ederer D.R., Carney L.S., Montgomery D.D., Lirette S.T., Marshall G.D. IgG Antibody Response to the Pfizer BNT162b2 SARS-CoV-2 Vaccine in Healthcare Workers with Healthy Weight, Overweight, and Obesity. Vaccines. 2022;10:512. doi: 10.3390/vaccines10040512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basilico S., Dubini C., Milani V., Bertolini C., Malavazos A.E. Could fat distribution have a greater influence than BMI on the antibody titre after SARS-CoV-2 vaccine? Obesity. 2022;30:1321–1322. doi: 10.1002/oby.23474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malavazos A.E., Basilico S., Iacobellis G., Milani V., Cardani R., Boniardi F., Dubini C., Prandoni I., Capitanio G., Renna L.V., et al. Antibody responses to BNT162b2 mRNA vaccine: Infection-naïve individuals with abdominal obesity warrant attention. Obesity. 2022;30:606–613. doi: 10.1002/oby.23353. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe M., Balena A., Tuccinardi D., Tozzi R., Risi R., Masi D., Caputi A., Rossetti R., Spoltore M.E., Filippi V., et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab. Res. Rev. 2022;38:e3465. doi: 10.1002/dmrr.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaborit B., Fernandes S., Loubet P., Ninove L., Dutour A., Cariou B., Coupaye M., Clement K., Czernichow S., Carette C., et al. Early humoral response to COVID-19 vaccination in patients living with obesity and diabetes in France. The COVPOP OBEDIAB study with results from the ANRS0001S COV-POPART cohort. Metabolism. 2023;142:155412. doi: 10.1016/j.metabol.2023.155412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tartof S.Y., Slezak J.M., Fischer H., Hong V., Ackerson B.K., Ranasinghe O.N., Frankland T.B., Ogun O.A., Zamparo J.M., Gray S., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., Doolman R., Asraf K., Mendelson E., Ziv A., et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N. Engl. J. Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shrotri M., Navaratnam A.M., Nguyen V., Byrne T., Geismar C., Fragaszy E., Beale S., Fong W.L.E., Patel P., Kovar J., et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet. 2021;398:385–387. doi: 10.1016/S0140-6736(21)01642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lustig Y., Gonen T., Meltzer L., Gilboa M., Indenbaum V., Cohen C., Amit S., Jaber H., Doolman R., Asraf K., et al. Superior immunogenicity and effectiveness of the third compared to the second BNT162b2 vaccine dose. Nat. Immunol. 2022;23:940–946. doi: 10.1038/s41590-022-01212-3. [DOI] [PubMed] [Google Scholar]
- 21.Lombardi A., Consonni D., Oggioni M., Bono P., Renteria S.U., Piatti A., Pesatori A.C., Castaldi S., Muscatello A., Riboldi L., et al. SARS-CoV-2 anti-spike antibody titres after vaccination with BNT162b2 in naïve and previously infected individuals. J. Infect. Public Health. 2021;14:1120–1122. doi: 10.1016/j.jiph.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manisty C., Otter A.D., Treibel T.A., McKnight Á., Altmann D.M., Brooks T., Noursadeghi M., Boyton R.J., Semper A., Moon J.C. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397:1057–1058. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krammer F., Srivastava K., Alshammary H., Amoako A.A., Awawda M.H., Beach K.F., Bermúdez-González M.C., Bielak D.A., Carreño J.M., Chernet R.L., et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N. Engl. J. Med. 2021;384:1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ebinger J.E., Fert-Bober J., Printsev I., Wu M., Sun N., Prostko J.C., Frias E.C., Stewart J.L., Van Eyk J.E., Braun J.G., et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021;27:981–984. doi: 10.1038/s41591-021-01325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anichini G., Terrosi C., Gandolfo C., Gori Savellini G., Fabrizi S., Miceli G.B., Cusi M.G. SARS-CoV-2 Antibody Response in Persons with Past Natural Infection. N. Engl. J. Med. 2021;385:90–92. doi: 10.1056/NEJMc2103825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neeland I.J., Ross R., Després J.-P., Matsuzawa Y., Yamashita S., Shai I., Seidell J., Magni P., Santos R.D., Arsenault B., et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019;7:715–725. doi: 10.1016/S2213-8587(19)30084-1. [DOI] [PubMed] [Google Scholar]
- 27.Pérez-Alós L., Armenteros J.J.A., Madsen J.R., Hansen C.B., Jarlhelt I., Hamm S.R., Heftdal L.D., Pries-Heje M.M., Møller D.L., Fogh K., et al. Modeling of waning immunity after SARS-CoV-2 vaccination and influencing factors. Nat. Commun. 2022;13:1614. doi: 10.1038/s41467-022-29225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdollahi A., Afsharyzad Y., Vaezi A., Meysamie A. Importance of the COVID-19 Vaccine Booster Dose in Protection and Immunity. Vaccines. 2022;10:1708. doi: 10.3390/vaccines10101708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe M., Balena A., Masi D., Tozzi R., Risi R., Caputi A., Rossetti R., Spoltore M.E., Biagi F., Anastasi E., et al. Rapid Weight Loss, Central Obesity Improvement and Blood Glucose Reduction Are Associated with a Stronger Adaptive Immune Response Following COVID-19 mRNA Vaccine. Vaccines. 2022;10:79. doi: 10.3390/vaccines10010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Renna L.V., Bertani F., Podio A., Boveri S., Carrara M., Pinton A., Milani V., Spuria G., Nizza A.F., Basilico S., et al. Impact of BNT162b2 Booster Dose on SARS-CoV-2 Anti-Trimeric Spike Antibody Dynamics in a Large Cohort of Italian Health Care Workers. Vaccines. 2023;11:463. doi: 10.3390/vaccines11020463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Golec M., Zembala-John J., Fronczek M., Konka A., Bochenek A., Wystyrk K., Botor H., Zalewska M., Chrapiec M., Kasperczyk S., et al. Relationship between anthropometric and body composition parameters and anti-SARS-CoV-2 specific IgG titers in females vaccinated against COVID-19 according to the heterologous vaccination course: A cohort study. PLoS ONE. 2023;18:e0287128. doi: 10.1371/journal.pone.0287128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glück V., Tydykov L., Mader A.-L., Warda A.-S., Bertok M., Weidlich T., Gottwald C., Köstler J., Salzberger B., Wagner R., et al. Humoral immunity in dually vaccinated SARS-CoV-2-naïve individuals and in booster-vaccinated COVID-19-convalescent subjects. Infection. 2022;6:e0287128. doi: 10.1007/s15010-022-01817-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herzberg J., Fischer B., Lindenkamp C., Becher H., Becker A.-K., Honarpisheh H., Guraya S.Y., Strate T., Knabbe C. Persistence of Immune Response in Health Care Workers After Two Doses BNT162b2 in a Longitudinal Observational Study. Front. Immunol. 2022;13:839922. doi: 10.3389/fimmu.2022.839922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grant R.W., Dixit V.D. Adipose tissue as an immunological organ. Obesity. 2015;23:512–518. doi: 10.1002/oby.21003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fasshauer M., Blüher M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015;36:461–470. doi: 10.1016/j.tips.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 36.Romacho T., Elsen M., Röhrborn D., Eckel J. Adipose tissue and its role in organ crosstalk. Acta Physiol. 2014;210:733–753. doi: 10.1111/apha.12246. [DOI] [PubMed] [Google Scholar]
- 37.Fan X., Han J., Zhao E., Fang J., Wang D., Cheng Y., Shi Y., Wang Z., Yao Z., Lu P., et al. The effects of obesity and metabolic abnormalities on severe COVID-19-related outcomes after vaccination: A population-based study. Cell Metab. 2023;35:585–600.e5. doi: 10.1016/j.cmet.2023.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frasca D., Reidy L., Cray C., Diaz A., Romero M., Kahl K., Blomberg B.B. Influence of obesity on serum levels of SARS-CoV-2-specific antibodies in COVID-19 patients. PLoS ONE. 2021;16:e0245424. doi: 10.1371/journal.pone.0245424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kara Z., Akçin R., Demir A.N., Dinç H.Ö., Taşkın H.E., Kocazeybek B., Yumuk V.D. Antibody Response to SARS-CoV-2 Vaccines in People with Severe Obesity. Obes. Surg. 2022;32:2987–2993. doi: 10.1007/s11695-022-06181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahn S.-Y., Sohn S.-H., Lee S.-Y., Park H.-L., Park Y.-W., Kim H., Nam J.-H. The effect of lipopolysaccharide-induced obesity and its chronic inflammation on influenza virus-related pathology. Environ. Toxicol. Pharmacol. 2015;40:924–930. doi: 10.1016/j.etap.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 41.Ryan P.M., Caplice N.M. Is Adipose Tissue a Reservoir for Viral Spread, Immune Activation, and Cytokine Amplification in Coronavirus Disease 2019? Obesity. 2020;28:1191–1194. doi: 10.1002/oby.22843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A., Labreuche J., Mathieu D., Pattou F., Jourdain M., et al. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity. 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalligeros M., Shehadeh F., Mylona E.K., Benitez G., Beckwith C.G., Chan P.A., Mylonakis E. Association of Obesity with Disease Severity Among Patients with Coronavirus Disease. Obesity. 2020;28:1200–1204. doi: 10.1002/oby.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klang E., Kassim G., Soffer S., Freeman R., Levin M.A., Reich D.L. Severe Obesity as an Independent Risk Factor for COVID-19 Mortality in Hospitalized Patients Younger than 50. Obesity. 2020;28:1595–1599. doi: 10.1002/oby.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wajnberg A., Mansour M., Leven E., Bouvier N.M., Patel G., Firpo-Betancourt A., Mendu R., Jhang J., Arinsburg S., Gitman M., et al. Humoral response and PCR positivity in patients with COVID-19 in the New York City region, USA: An observational study. Lancet Microbe. 2020;1:e283–e289. doi: 10.1016/S2666-5247(20)30120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soffer S., Glicksberg B.S., Zimlichman E., Efros O., Levin M.A., Freeman R., Reich D.L., Klang E. The association between obesity and peak antibody titer response in COVID-19 infection. Obesity. 2021;29:1547–1553. doi: 10.1002/oby.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wrigley Kelly N.E., Kenny G., Cassidy F.C., Garcia-Leon A.A., De Barra C., Mallon P.W., Hogan A.E., O’Shea D. Individuals with obesity who survive SARS-CoV-2 infection have preserved antigen-specific T cell frequencies. Obesity. 2022;30:1927–1931. doi: 10.1002/oby.23526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muena N.A., García-Salum T., Pardo-Roa C., Avendaño M.J., Serrano E.F., Levican J., Almonacid L.I., Valenzuela G., Poblete E., Strohmeier S., et al. Induction of SARS-CoV-2 neutralizing antibodies by CoronaVac and BNT162b2 vaccines in naïve and previously infected individuals. eBioMedicine. 2022;78:103972. doi: 10.1016/j.ebiom.2022.103972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pilz S., Theiler-Schwetz V., Trummer C., Krause R., Ioannidis J.P. SARS-CoV-2 reinfections: Overview of efficacy and duration of natural and hybrid immunity. Environ. Res. 2022;209:112911. doi: 10.1016/j.envres.2022.112911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldberg Y., Mandel M., Bar-On Y.M., Bodenheimer O., Freedman L.S., Ash N., Alroy-Preis S., Huppert A., Milo R. Protection and Waning of Natural and Hybrid Immunity to SARS-CoV-2. N. Engl. J. Med. 2022;386:2201–2212. doi: 10.1056/NEJMoa2118946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flury B.B., Güsewell S., Egger T., Leal O., Brucher A., Lemmenmeier E., Kleeb D.M., Möller J.C., Rieder P., Rütti M., et al. Risk and symptoms of COVID-19 in health professionals according to baseline immune status and booster vaccination during the Delta and Omicron waves in Switzerland-A multicentre cohort study. PLoS Med. 2022;19:e1004125. doi: 10.1371/journal.pmed.1004125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.