Figure 3.
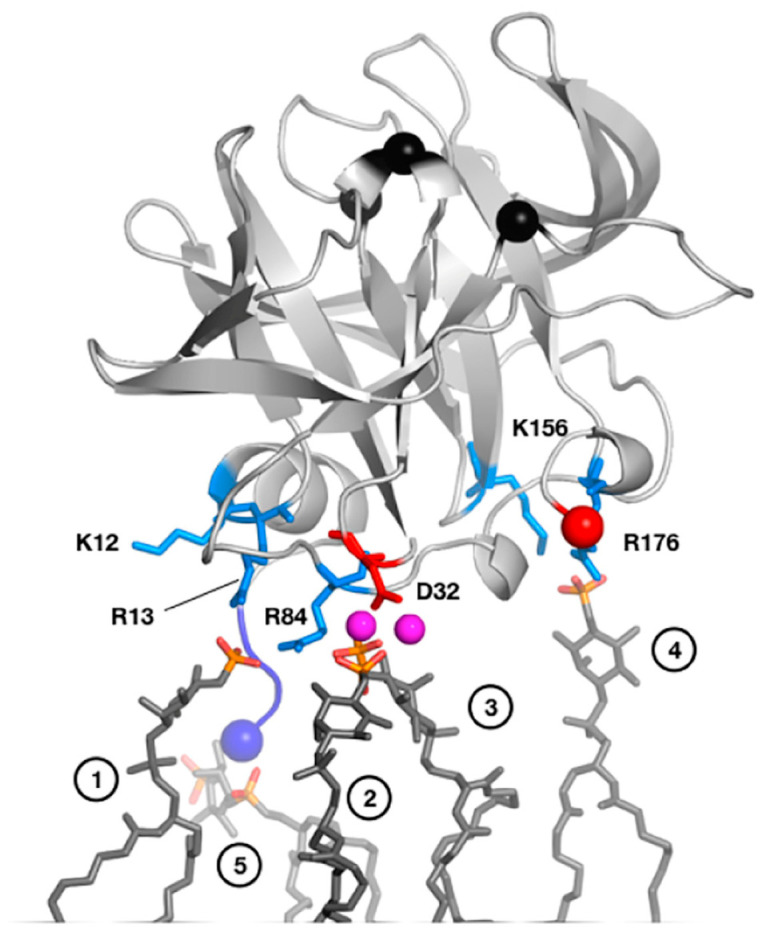
MD-derived model of PV 3C interactions with a PI4P-containing lipid membrane. Residues involved in interactions include Arg13 in the N-terminal alpha helix and Arg84 in the RNA-binding region. NMR studies with short-chain, soluble PI4P lipids largely confirm this model. In this model, PV 3C interacts with five clustered PI4P lipid molecules as shown in dark grey sticks with phosphates colored orange (phosphorus) and red (oxygen). The following specific interactions are listed, with numbers in parenthesis denoting the panel’s head group: (1) R13 and R84; (2) D32, mediated by sodium ions; (3) D32 mediated by sodium; (4) K156 and R176; and (5) α-amino group of G1. Reprinted from Structure, 25(12), D. Shengjuler, Y.M. Chan, S. Sun, I. Moustafa, Z.-L. Li, D.W. Gohara, M. Buck, P.S. Cremer, D.D. Boehr, C.E. Cameron. The RNA-binding site of poliovirus 3C protein doubles as a phosphoinositide-binding domain, 1875–1886, Copyright (2017) with permission from Elsevier.
