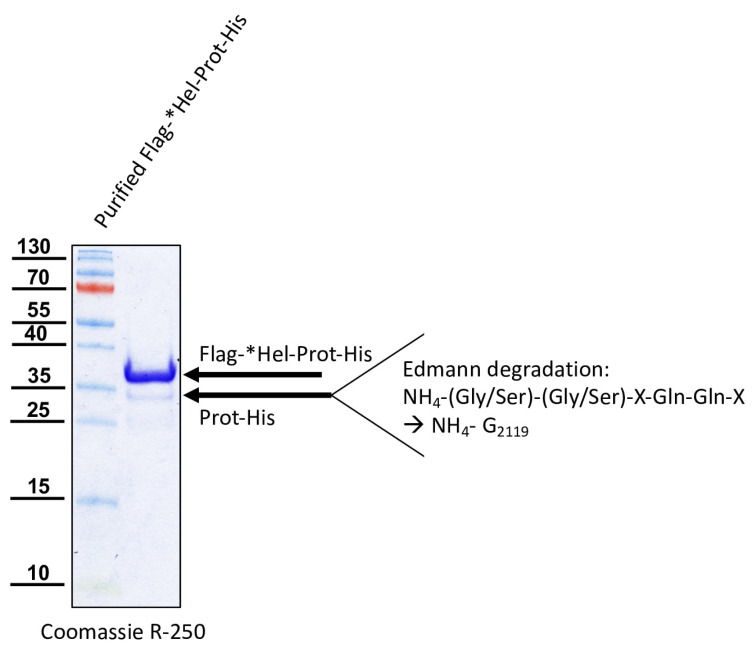
Figure 9.
N-terminal sequencing of the purified Prot-His fragment uncovered the N-terminal site of processing. Flag-*Hel-Prot-His was purified via IMAC and subjected to SDS-PAGE. The Coomassie stained gel showed the unprocessed Flag-*Hel-Prot-His (38 kDa) and the cleaved Prot-His fragment (35 kDa). After blotting onto a PVDF membrane and staining, the Pol*-His fragment was excised and analyzed via Edmann degradation. Again, the small amount of protein in the 35 kDa band led to a limited number of evaluable degradation cycles. However, a sequence with amino acids glycine or serine (step 1), glycine or serine (step 2), an unassignable amino acid (step 3), and two glutamines (step 4 and step 5) could be clearly assigned to the cleavage position P1′ G2119.